Figure 6. Recognition requirements for high-entropy residues contribute to strain specificity of 2C06 and 2C09.
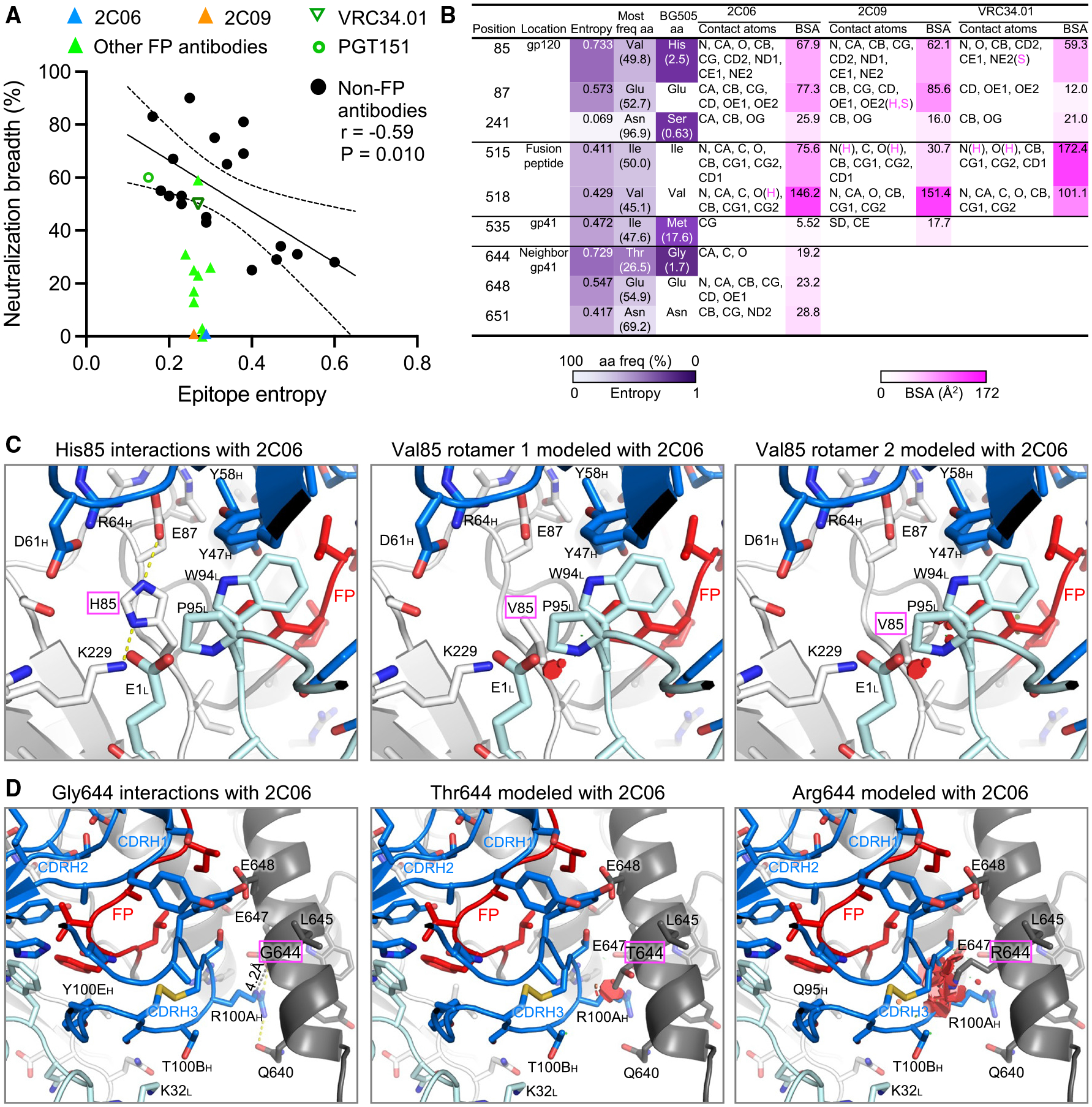
(A) While neutralization breadth generally correlates with epitope entropy, antibodies directed to the fusion-peptide sites of vulnerability have similar epitope entropy. Neutralization breadth represents the percentage of the 208-strain panel neutralized with IC50 < 50 μg/mL. Epitope entropy was calculated as BSA-weighted average of normalized Shannon’s entropy on the Env sequences of the 208-strain panel.
(B) Analyses of sequence entropy and contact surface areas of residues in strain-specific and broadly neutralizing fusion-peptide-directed antibodies. Epitope residues with normalized entropy above 0.4 are listed, as well as residue S241, which has low entropy but with low frequency as serine among HIV-1 isolates. The contact atoms and areas for VRC34.01 were calculated from PDB: 5i8h (VRC34.01 also binds AMC011 Env, which has valine85). Entropy values were calculated with Shannon Entropy-One (https://www.hiv.lanl.gov/content/sequence/ENTROPY/entropy_one.html) on the curated alignment of year 2020 HIV-1 M group Env of 5,255 sequences (https://www.hiv.lanl.gov/content/sequence/NEWALIGN/align.html), and were normalized to have values between 0 and 1. Contact atoms and BSA were calculated with PISA (https://www.ebi.ac.uk/msd-srv/prot_int/cgi-bin/piserver). Hydrogen bonds or salt bridges involved with the contact atoms are designated as H or S, respectively.
(C) H85, with high Shannon entropy of 0.733 and 2.5% frequency, has tight interactions with antibody heavy- and light-chain residues in both structures of the 2C06 complex (2C09 has nearly identical interactions, see Figure S6). Modeling in PyMOL with the most frequent amino acid valine results in clashes with CDR L3, either P95 or W94 or both, with any rotamers. The second most common amino acid at 85 is glutamate at 8.5% frequency and would have severe clashes with the antibody. (D) Antibody 2C06 had close contact with G644 from its R100A side chain of CDR H3, which played a major role in binding fusion peptide. Modeling as threonine (26.5% frequency) or arginine (20.1%) would result in clashes, disrupting CDR H3 interactions with fusion peptide.
See also Figures S2 and S6.
