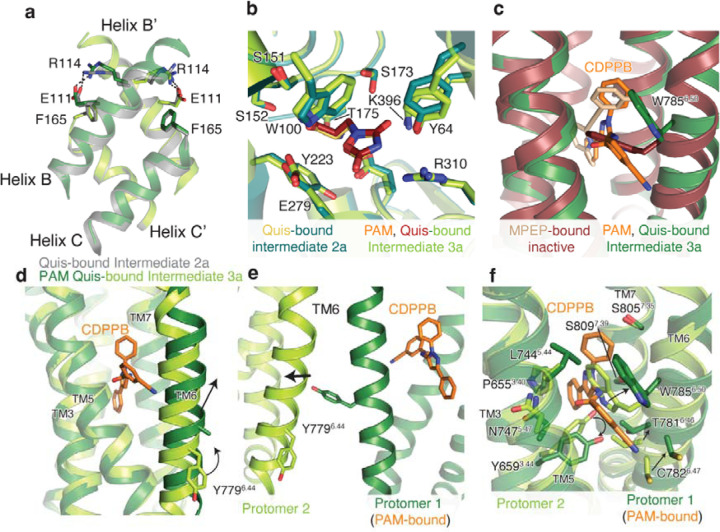
Extended Data Figure 9: CDPPB, Quis-bound structural analysis.
(a) Overlay of intersubunit B and C helices in Quis-bound Intermediate 2a state and CDPPB, Quis-bound Intermediate 3a structure. Residues R114 and E111 interact in both structures.
(b) Overlay of Quis binding pocket in Quis-bound Intermediate 2a and CDPPB, Quis-bound Intermediate 3a structures, showing no difference in the ligand pocket.
(c) The conformation of residue W7856.50 is different in the structure with the NAM, MPEP (PDB: 6FFI, brown) compared to that with the PAM, CDPPB (dark green).
(d) TM6 in the CDPPB-bound Protomer 1 has moved outward compared to Protomer 2 with no CDPPB bound. In CDPPB-bound Protomer 1, Y7796.44 points towards the intersubunit interface, as seen in (e). Though we cannot model the Y7796.44 sidechain in Protomer 1 with confidence due to a lack of good density, we have added the most frequently occurring rotomer of Tyr.
(f) Comparison of the allosteric pocket in CDPPB-bound protomer (protomer 1, dark green and CDPPB shown as orange) and the protomer with no CDPPB (protomer 2, green).