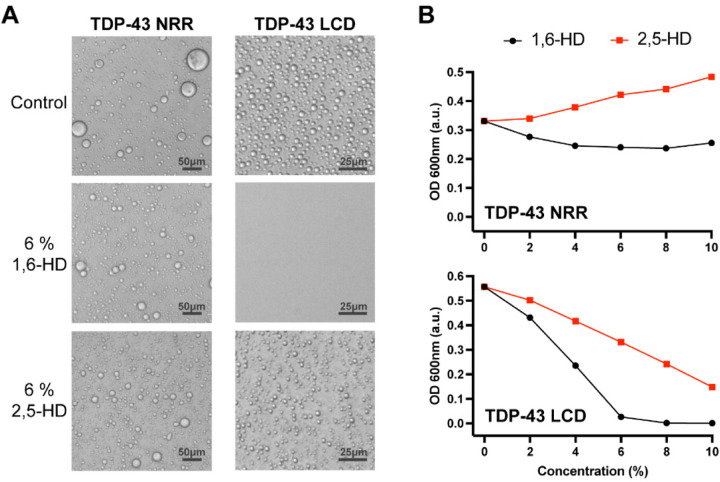
Fig. 1. Effects of aliphatic alcohols on phase separated droplets formed from the structured and unstructured halves of the TDP-43 RNA-binding protein.
(A) Protein fragments corresponding to residues 1–262 or 263–414 of the TDP-43 protein were expressed in bacteria, purified and incubated under conditions of neutral pH and physiologically normal monovalent salt ions. Both the N-terminal fragment (residues 1–262) bearing the structured N-terminal oligomerization domain and two RRM domains (NRR), and the C-terminal low complexity domain (LCD, residues 263–414) became phase separated in the form of spherical protein droplets under a protein concentration of 15 to 20 μM. 4% PEG-8,000 was added to the purified NRR to facilitate formation of liquid-like droplets. Such droplets were immune to the effects of both 1,6-hexanediol (1,6-HD) and the regioisomeric 2,5-hexanediol (2,5-HD) chemical. Droplets formed from the C-terminal LCD were fully melted upon exposure to 6% 1,6-HD, but left largely intact upon exposure to 6% 2,5-HD. (B) Phase separation by the TDP-43 NRR and LCD was quantified by measurements of turbidity. Neither aliphatic alcohol caused a reduction in turbidity of the sample formed by the TDP-43 NRR (upper panel). At all concentrations tested, 1,6-HD reduced turbidity more substantially than 2,5-HD for droplets formed from the TDP-43 LCD (lower panel).