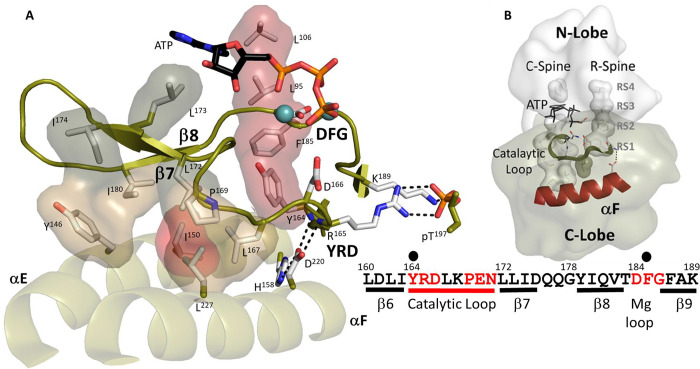
Figure 2. Hydrophobic interface anchors the Catalytic machinery to the αE-helix and αF-helix.
(A). I150 (in red) from αE-helix plays an important role by docking to αF-helix and Catalytic loop. Three residues from β7, L172, L173, and I174 (in dark tan) assemble the hydrophobic surface from αE-helix to ATP pocket. L173 and I174 are part of C-Spine. R-spine are also shown in red. D220 bridges H158 from αE-helix to YRD motif. (B). The logo of spines shows how important those hydrophobic residues are. The sequence of β6-β9 segment is also shown.