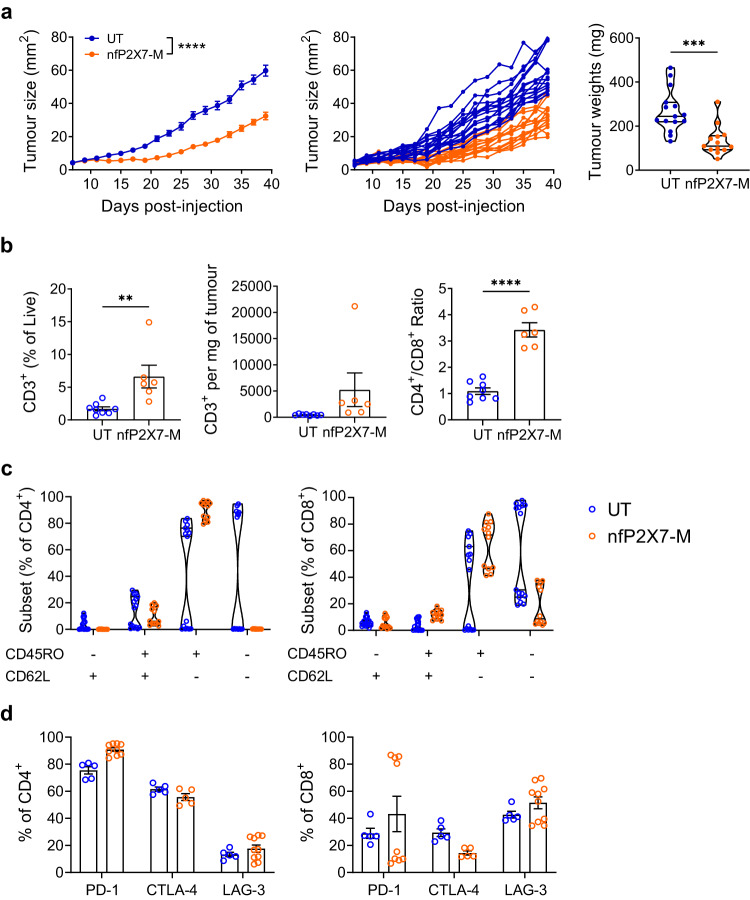
Fig. 6. NfP2X7-targeting CAR-T cells significantly inhibit the tumourigenesis of a human breast cancer xenograft model.
6–8-week-old female NOD-scid IL2Rγnull (NSG) mice were subcutaneously injected with 2 × 106 MDA-MB-231 human breast cancer cells into the fourth mammary fat pad and intravenously injected with 2 × 107 nfP2X7-targeting CAR-T cells or untransduced T cells on d3 post-tumour injection. Tumours were harvested for flow cytometric analysis at d40 post-tumour injection. a Tumour growth curves (as pooled and individual mice) and endpoint tumour weights; n = 15 (UT) and n = 13 (nfP2X7-M). Tumour size: two-way ANOVA with Bonferroni’s post-test, ****p < 0.0001; tumour weight: two-tailed unpaired t-test, ***p = 0.0003. b Frequency of human CD3+ T cells of total viable cells in tumours, number of human CD3+ T cells per mg of tumour and CD4+/CD8+ ratio of CD3+; n = 8 (UT) and n = 6 (nfP2X7-M); two-tailed unpaired t-test, **p = 0.0071, ****p < 0.0001. c Frequencies of T cell subsets as defined by CD45RO and CD62L expression by intratumoural CD4+ and CD8+ T cells; n = 15 (UT) and n = 12 (nfP2X7-M); two-way ANOVA with Bonferroni’s post-test. d Frequency of PD-1, CTLA-4 and LAG-3 expression by intratumoural CD4+ and CD8+ T cells; n = 5 (UT PD-1, CTLA-4, LAG-3) and n = 9 (nfP2X7-M PD-1), n = 5 (nfP2X7-M CTLA-4), n = 10 (nfP2X7-M LAG-3). Data in (a, c, d) are pooled from 2 independent experiments following in vivo delivery of 2 independent CAR-T cell preparations derived from 1 healthy donor and data in (b) representative of a single independent experiment. Data represented as mean ± SEM.