Abstract
Background
The PD-L1 on tumor cell-derived small extracellular vesicles (sEVs) can suppress the proliferation and cytokine production of T cells. However, PD-L1 can also be expressed by non-tumor cells. The present study is designed to test whether immunocytes release immunosuppressive PD-L1-positive sEVs.
Methods
sEVs were isolated from different clinical samples of head and neck squamous cell carcinoma (HNSCC) patients, the level and cellular origins of PD-L1-positive sEVs were assessed. Co-expression of CD80 on PD-L1-positive sEVs was examined to evaluate the immunosuppressive and tumor-promotive effects.
Results
PD-L1-positive sEVs in HNSCC patients had various cellular origins, including tumor cell, T cell, B cell, dendritic cell and monocyte/macrophage. However, PD-L1-positive sEVs derived from immune cells did not exert immunosuppressive functions due to the co-expression of CD80. It was verified that co-expression of CD80 disrupted the binding of sEV PD-L1 to its receptor PD-1 on T cells and attenuated the immunosuppression mediated by sEV PD-L1 both in vitro and in vivo.
Conclusion
The study suggests that PD-L1-positive sEVs have the cellular origin and functional heterogeneity. Co-expression of CD80 could restrict the immunosuppressive effect of sEV PD-L1. A greater understanding of PD-L1-positive sEV subsets is required to further improve their clinical application.
Subject terms: Cancer immunotherapy, Oral cancer detection
Introduction
Programmed cell death protein-ligand 1 (PD-L1) is an immune checkpoint molecule that binds to programmed cell death protein-1 (PD-1) and negatively regulates the activation and function of immunocytes [1]. Conventional PD-L1/PD-1-mediated immunosuppression is based on the physical contact between tumor cells and T cells in the tumor microenvironment. However, recent reports, including our study, have revealed that exosomal PD-L1 secreted by tumor cells can systemically counter the antitumor immunity [2, 3]. Small extracellular vesicles (sEVs, <200 nm in diameter), including exosomes, are naturally occurring membrane vesicles that can carry and transport biological molecules between cells acting as critical intercellular communicators that regulate various pathophysiological processes, including immunosuppression [4]. The level of PD-L1 on circulating EVs/exosomes in cancer patients has been suggested as a potential predictor of clinical response [3, 5, 6]. These findings provide a rationale for the future application of sEV PD-L1-based liquid biopsy, and the role of sEV PD-L1 in antitumor immunity is a promising new research focus. Nevertheless, further investigations are required to uncover the mechanisms, cellular origins and functions of PD-L1-positive sEVs and improve their diagnostic and predictive performance.
PD-L1-positive sEVs are mostly secreted by tumor cells, and PD-L1 expression can be enhanced by procedures such as IFN-γ stimulation or radiation [3, 7]. However, PD-L1 can also be expressed by mesenchymal stem cells and immunocytes, such as T cells, natural killer cells and dendritic cells (DCs) [8–10]. Thus, it is possible that these PD-L1-expressing non-tumor cells also secrete PD-L1-positive sEVs. Li and colleagues recently demonstrated that PD-L1 was enriched on mesenchymal stem cell-derived sEVs [8]. Another study in patients with head and neck squamous cell carcinoma (HNSCC) revealed that PD-L1 could be detected on CD3-positive exosomes, indicating a T-cell origin [11]. Serratì et al. used CD146, CD8, CD19, CD14 and CD1a as markers for identifying cellular origins, they revealed and quantified PD-L1-positive sEVs secreted by melanoma cells, CD8+ T cells, B cells, monocytes and DCs in melanoma patients [6]. Overall, PD-L1-positive sEVs appear to be secreted by different cell types, resulting in the source heterogeneity of PD-L1-positive sEVs. These findings raise the question of whether PD-L1-positive sEVs secreted by non-tumor cells can also exert immunosuppressive functions. Although there is no clear answer to this question, the available results have indicated distinct performance between tumor and non-tumor cell-derived PD-L1-positive sEVs, especially those from immunocytes, in disease diagnosis and prediction. Recent studies indicate that PD-L1 and its partners, CD80 and/or PD-1, can be co-expressed on the same surface of immunocytes, and CD80 or PD-1 can interact with PD-L1 in cis to disrupt PD-L1/PD-1 binding in trans between antigen-presenting cells and T cells. Subsequently, the effect of PD-L1-induced T cell suppression can be restricted by CD80 or PD-1 [12, 13]. We hypothesize that PD-L1-positive sEVs derived from immunocytes may also contain CD80 and/or PD-1 and that their immunosuppressive role would be affected by this co-expression.
In our present study, we comprehensively assessed the cellular origins of PD-L1-positive sEVs from different clinical samples, including plasma and tumor tissue from HNSCC patients and plasma from healthy donors (HD). Tumor cell-derived sEVs (T-sEVs) and immune cell-derived sEVs (I-sEVs) exhibited different clinical significance in HNSCC, and showed distinct functions on T cells. Further investigations demonstrated that the co-expression of CD80 on the I-sEVs disrupted the binding of sEV PD-L1 to its receptor PD-1 on T cells and attenuated the immunosuppression mediated by sEV PD-L1 both in vitro and in vivo. In summary, our findings provide a better understanding of the heterogeneity of PD-L1-positive sEVs, which may help to improve the clinical application of PD-L1-positive sEVs.
Materials and methods
Clinical sample collection
Blood samples and tumor tissue samples of 86 patients with HNSCC were collected from the Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Wuhan University and Zhongnan Hospital of Wuhan University. Among them, 37 patients with paired samples were analyzed for the difference of PD-L1-positive sEVs between HNSCC patients and healthy donors (HD), and the correlation of PD-L1-positive sEV subsets with clinical characteristics; 27 patients with paired samples were analyzed for the distributions of PD-L1-positive sEV subsets in blood and the correlation between PD-L1-positive sEV subsets and PD-L1 expression in tumor tissues; 22 patients with recurrent and/or metastatic HNSCC treated with anti-PD-1 therapy were analyzed for the correlation between PD-L1-positive sEV subsets and therapy response (assessed according to immune-related Response Evaluation Criteria in Solid Tumors, irRECIST) [14]. The information on the individuals is shown in Supplementary Table 1. Blood samples were collected from 35 HD (16 females and 19 males) with a median age of 36 years for sEV isolation and primary immune cell generation.
Isolation and characterization of sEVs
The detailed methods for the isolation and characterization of sEVs from plasma, tumor tissues and in vitro cultured cell lines are provided in the Supplementary Methods. To isolate sEVs from tumor tissues, fresh tissues were cut into small fragments and added to dissociation buffer (1 mg/ml papain; 5.5 mM l-cysteine; 67 μM 2-mercaptoethanol; 1.1 mM EDTA) at 37 °C for 20 min, the samples were further digested using a tissue homogenizer (Miltenyi gentleMACS Dissociator, Germany). Then the sEVs were purified by differential centrifugation and characterized using a transmission electron microscope (Hitachi HT-7700, Japan). The size distribution and concentration of sEVs were analyzed on a ZetaView (Merkel Technologies Ltd., Germany).
ELISA and western blot analysis
The methods of ELISA test and western blot analysis for sEVs were described in our previous study [3]. Detailed methods are provided in the Supplementary Methods. The primary antibodies are shown in Supplementary Table 2.
Flow cytometry
Cells were stained with indicated antibodies. Cell surface staining was performed for 30 min at 4 °C, and the intracellular staining was performed for 60 min on ice after using a fixation/permeabilization kit (eBioscience, Cat# 00-5521-00, USA). Stained cells were analyzed by CytoFLEX (Beckman, USA). For high-resolution flow cytometry, purified sEVs were stained with indicated antibodies for 60 min at 4 °C and then ultra-centrifugated at 120,000 × g for 1 h at 4 °C (Beckman Optima MAX-XP, USA). The pelleted sEVs were resuspended by PBS and analyzed by A60-Micro PLUS (Apogee, UK). Given that the steric hindrance may limit the binding of antibodies to sEVs [15], sEVs were stained with no more than three antibodies at a time. The data were analyzed by Flowjo v10.0 (TreeStar, USA). The used primary antibodies are shown in Supplementary Table 2.
Soluble protein binding assay
To test the binding capacity of PD-L1 on sEVs to soluble PD-1 protein (Sinobiological, Cat# 10377-H02H, China) or PD-1/CD80 on sEVs to soluble PD-L1 protein (Sinobiological, Cat# 10084-H02H, China), 100 μl of sEVs were incubated with 4 μg/ml human Fc-tagged protein overnight at 4 °C, then the Fc-tag was stained by Fc PE immunofluorescent antibody. The unbound protein and antibody were removed by ultra-centrifuged at 120,000 × g for 1 h (Beckman Optima XPN, USA). The pelleted sEVs were resuspended and analyzed by high-resolution flow cytometry.
Treatment of T cells with PD-L1-positive sEVs
To test the effects of PD-L1-positive sEVs on Jurkat T cells, Jurkat cells were incubated with a total of 0.9 ng/ml sEV PD-L1 (the mean level of sEV PD-L1 in HNSCC patients). For this purpose, 22.5 μg/ml of CAL27 sEVs (0.04 ng PD-L1 per μg of sEVs), 20 μg/ml of H1975 sEVs (0.045 ng PD-L1 per μg of sEVs), 45 μg/ml of Raji sEVs (0.020 ng PD-L1 per μg of sEVs) and 300 μg/ml of THP-1 sEVs (0.003 ng PD-L1 per μg of sEVs) were used, respectively. The sEVs were pre-stained with carboxyfluorescein succinimidyl ester (CFSE) and pelleted by differential centrifugation. Jurkat cells were then prepared for confocal microscopy or flow cytometry. For confocal microscopy, Jurkat cells were stained with DAPI and phalloidin (ThermoFisher Scientific, USA) and then observed using a spinning-disk confocal microscope system (The Andor Revolution XD).
In vivo mice study
PD-L1KO B16F10 cells (2 × 105 cells) or MC38 cells (5 × 105 cells) were injected into the back of immunocompetent C57BL/6 mice (female, 7–8 weeks). Then the mice were randomly divided into research groups. PBS or 50 μg of sEVs (diluted in 100 μl PBS) were injected through the tail vein 10 days after implantation, injections were then performed every 2 days and the mice were weighted. The dose of 50 μg sEVs applied for the in vivo study was equivalent to approximately 15% of physiological level of circulating sEVs in mice [3, 6]. Tumor volume was calculated by the formula: length × (width)2/2. For flow cytometry, the spleen, blood, inguinal lymph nodes and tumor samples were harvested for single-cell suspension preparation.
Statistical analysis
All data were analyzed with GraphPad Prism v.8.0. D’Agostino-Pearson omnibus normality test was used to determine normality of distribution and the F-test was performed for calculating variance between groups. Paired or unpaired Student’s t-test (two groups), or one-way ANOVA (more than two groups) was performed to analyze differences between groups in normally distributed data. A nonparametric unpaired Mann–Whitney test was performed to determine differences in abnormally distributed data. Correlation analysis of two parameters was performed using Pearson’s r coefficient. Receiver operating characteristic (ROC) curves were conducted to assess the sensitivity, specificity, and cut-off values. Two-way ANOVA was used to compare tumor growth of different groups. Log-rank test was performed for assessing overall survival.
Results
Presence of PD-L1-positive sEVs in healthy donors
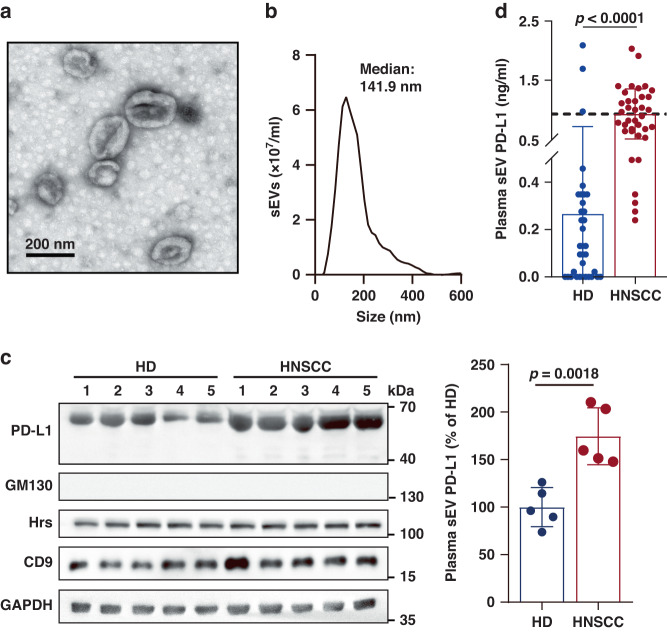
To confirm whether non-tumor cells could secrete PD-L1-positive sEVs into circulation, we purified sEVs from the plasma of both healthy donors (HD) and HNSCC patients by differential centrifugation. The cup-shaped morphology and size distribution (<200 nm) of sEVs were verified by transmission electron microscopy (TEM) (Fig. 1a) and nanoparticle tracking analysis (NTA) (Fig. 1b), respectively. The following markers were used for western blot analysis: Tetraspanins (e.g., CD9, CD81), members of a superfamily of proteins with four transmembrane domains and are abundant on sEV membranes; Hrs, a member of the endosomal sorting complex required for transport (ESCRT) family that regulates the cargo sorting of sEVs; GAPDH, a cytoplasmic glycolytic enzyme presents in both cells and sEVs but exhibits a higher enrichment in cells than sEVs; GM130, a Golgi marker as the negative control for sEVs (Fig. 1c). Although the level of sEV PD-L1 was lower in HD than HNSCC patients, sEV PD-L1 could still be detected in HD (Fig. 1c). Similar result was obtained using ELISA (Fig. 1d). The mean concentrations of sEV PD-L1 in HD and HNSCC patients were 0.27 ng/ml and 0.94 ng/ml, respectively. Notably, the result showed that sEV PD-L1 was detectable in 68.57% of HD (24/35). The presence of PD-L1-positive sEVs in HD indicates that non-tumor cells could also secrete PD-L1-positive sEVs.
Fig. 1. Presence of PD-L1-positive sEVs in both HD and HNSCC patients.
a A representative TEM image of purified circulating sEVs from HNSCC patients. b Characterization of purified circulating sEVs using NTA. c Western blot analysis of PD-L1 and sEV markers (CD9, Hrs and GAPDH; GM130, negative marker) in circulating sEVs purified from HD (n = 5) and HNSCC patients (n = 5). All lanes were loaded with the same amount of total protein (left). Quantification of the level of sEV PD-L1 as detected by western blotting (right). Results are expressed as percentages of the mean value of HD. The results shown represent three independent experiments. d ELISA data of PD-L1 on circulating sEVs isolated from HD (n = 35) and HNSCC patients (n = 37). The dotted line shows the mean level of sEV PD-L1 in HNSCC patients (0.94 ng/ml). Data are mean ± SD (c, d). P values are from two-sided unpaired t test (c, d).
Abundant PD-L1-positive sEVs secreted by immunocytes
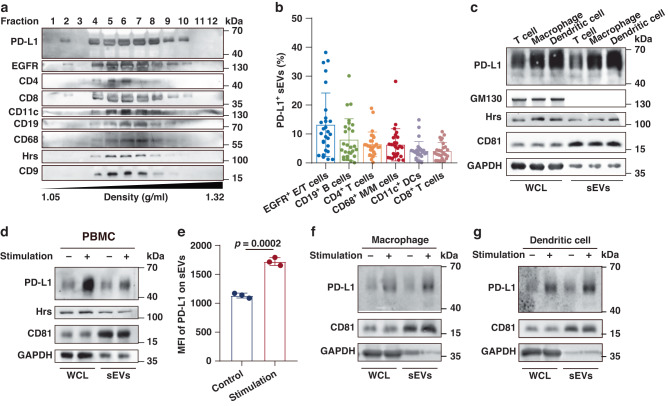
Next, sEVs were isolated from tumor tissue and plasma specimens of HNSCC patients to further confirm the heterogeneity of PD-L1-positive sEVs in terms of cellular origin. The mean number of sEVs isolated from plasma was 4.95 × 1010/ml (95% confidence interval, 2.18 × 1010/ml to 7.71 × 1010/ml), and the mean number of sEVs isolated from tumor tissue was 1.85 × 109/mg (95% confidence interval, 1.29 × 109/mg to 2.42 × 109/mg). The potential cellular origins of PD-L1-positive sEVs were analyzed using antibodies against different cell markers. Specifically, the expression of epidermal growth factor receptor (EGFR), a regulator of cell growth in tissues of epithelial origin, is often elevated in various cancers, including HNSCC [16, 17]. Western blot analysis of plasma sEVs purified by iodixanol density gradient centrifugation revealed the co-fraction of PD-L1 with sEV markers (Hrs and CD9), an epithelial/tumor cell marker (EGFR) and immunocyte markers (CD4, CD8, T cell; CD11c, DC; CD19, B cell; CD68, monocyte/macrophage) (Fig. 2a). We then defined the cellular origins of PD-L1-positive sEVs by co-expressing PD-L1 and cell markers using a high-resolution flow cytometer capable of detecting nanosized particles (Supplementary Fig. 1). Besides epithelial/tumor cell-derived PD-L1-positive sEVs, PD-L1-positive sEVs derived from various types of immunocytes could also be detected in both plasma and tumor tissue (Fig. 2b and Supplementary Fig. 2). Especially, the levels of B cell-, CD4+ T cell- and monocyte/macrophage-derived PD-L1-positive sEVs in plasma were determined as 8.01%, 6.42% and 6.27%, respectively (Fig. 2b). These results suggest that abundant PD-L1-positive sEVs in plasma may be released by immunocytes.
Fig. 2. PD-L1-positive sEVs secreted by immunocytes in patients and in vitro.
a Western blot analysis of PD-L1, cellular markers and sEV markers (Hrs, CD9) in sEVs isolated from HNSCC patients by iodixanol density gradient centrifugation. Cellular markers: EGFR (epithelial/tumor cells, E/T cells), CD4 (CD4+ T cells), CD8 (CD8+ T cells), CD11c (DCs), CD19 (B cells), and CD68 (monocyte/macrophages, M/M cells). Two experiments were repeated independently with similar results. b High-resolution flow cytometry of PD-L1-positive sEVs derived from E/T cells, B cells, CD4+ T cells, M/M cells, DCs and CD8+ T cells in plasma (n = 27). c Western blot analysis of PD-L1 in whole-cell lysates (WCL) and sEVs from PBMC-generated T cells, macrophages and dendritic cells. d Western blot analysis of PD-L1 in WCL and sEVs secreted by PBMCs with or without treatment with anti-CD3/CD28 antibodies. e Quantification for the mean fluorescence intensity (MFI) of PD-L1 on sEVs secreted by PBMCs with or without treatment with anti-CD3/CD28 antibodies, the MFI is tested by high-resolution flow cytometry. f, g Western blot analysis of PD-L1 in WCL and sEVs of LPS and IFN-γ stimulated macrophages and dendritic cells. The level of sEV PD-L1 derived from stimulated macrophages was 2.5 times as that from control macrophages, and the level of sEV PD-L1 derived from stimulated dendritic cells was 2.1 times as that from control dendritic cells. All lanes were loaded with the same amount of total protein (a, c, d, f, g). Results shown represent two (a) or three (c, d) independent experiments. GAPDH was set as the loading control for WCL, CD81 was set as the loading control for sEVs. P values are from two-sided unpaired t test (e).
To verify the above findings in patients, we then generated primary T cells, macrophages and DCs from peripheral blood mononuclear cells (PBMCs) and collected sEVs from the culture supernatant. The results showed the presence of PD-L1 in sEVs secreted by these primary immune cells (Fig. 2c). Moreover, we demonstrated that the level of PD-L1 on PBMC-derived sEVs was up-regulated by stimulating with anti-CD3/CD28 (Fig. 2d, e). When primary macrophages were stimulated with lipopolysaccharide (LPS) and primary DCs were stimulated with LPS plus IFN-γ, the levels of PD-L1 on their derived sEVs were greatly up-regulated (Fig. 2f, g). Similar results were obtained in a panel of human immune cell lines (T-cell leukemia Jurkat cells, acute monocytic leukemia THP-1 cells and Burkitt’s lymphoma Raji cells) and mouse cell lines (T lymphoma EL4 cells and dendritic cell DC2.4) (Supplementary Fig. 3). Together, these results demonstrate that immunocytes also secrete PD-L1-positive sEVs, and the level of PD-L1 can be significantly enhanced by stimulation of the parental cells.
Different clinical significance between tumor cell- and immunocyte-derived PD-L1-positive sEVs
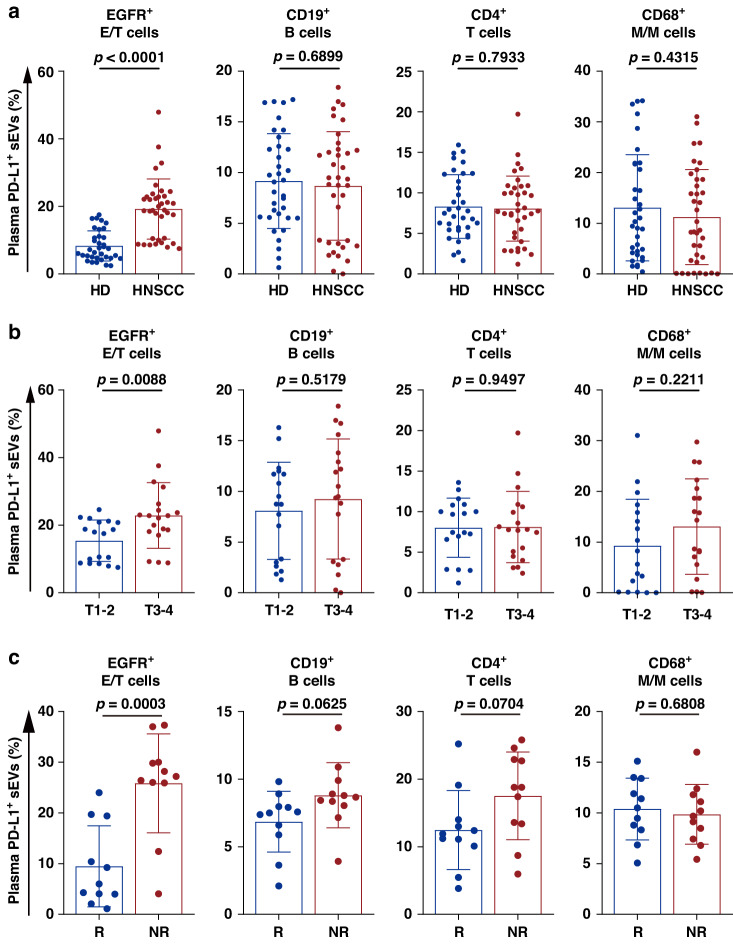
Next, we selected the top four subsets of PD-L1-positive sEVs in patients’ plasma to analyze their clinical roles in HNSCC. The results revealed that the proportion of epithelial/tumor cell-derived PD-L1-positive sEVs was significantly higher in HNSCC patients than that in HD, whereas there was no significant difference between HD and HNSCC patients in the proportions of PD-L1-positive I-sEVs (Fig. 3a). This result also showed that the proportion of PD-L1-positive sEVs derived from epithelial/tumor cells, but not that from immunocytes, in HNSCC patients with stage T3-4 was significantly higher than those with stage T1-2 (Fig. 3b). As an extension of our previous work, we further examined the correlation between the levels of PD-L1-positive sEV subsets and clinical response to anti-PD-1 therapy in 22 HNSCC patients, among whom 11 patients were responders and 11 patients were non-responders. The results showed that the pre-treatment level of epithelial/tumor cell-derived PD-L1-positive sEVs was most associated with the clinical response to anti-PD-1 therapy, the level of epithelial/tumor cell-derived PD-L1-positive sEVs was statistically lower in responders compared with non-responders (Fig. 3c). While there was no difference of PD-L1-positive I-sEVs between responders and non-responders. Taken together, these results suggest that PD-L1-positive sEVs derived from tumor cells or immunocytes may exert different functions.
Fig. 3. Different clinical significance between tumor cell- and immunocyte-derived PD-L1-positive sEVs.
a High-resolution flow cytometry of PD-L1-positive sEVs with various cellular origins in HD (n = 35) and HNSCC patients (n = 37). b High-resolution flow cytometry of PD-L1-positive sEVs with various cellular origins in patients with stage T1-2 (n = 18) and T3-4 (n = 19) HNSCC patients. c High-resolution flow cytometry of the pre-treatment level of plasma PD-L1-positive sEVs with different cellular origins in HNSCC patients treated with anti-PD-1 therapy. R, responders, n = 11; NR non-responders, n = 11. Data are mean ± SD. P values are from two-sided unpaired t test.
PD-L1-positive I-sEVs did not suppress T cells in vitro
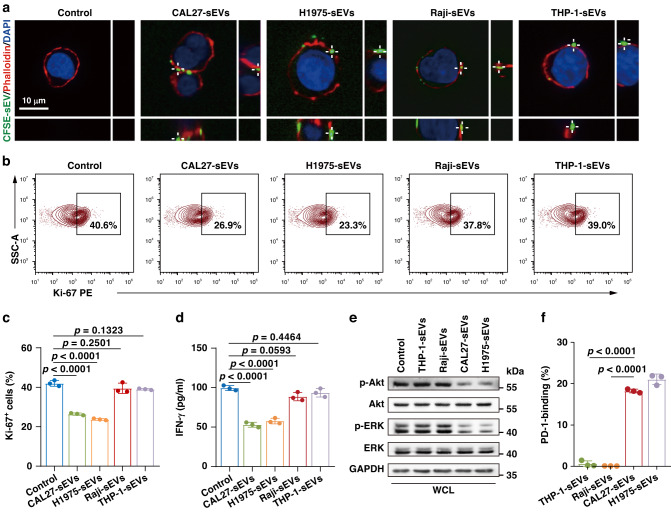
We previously demonstrated that PD-L1-positive T-sEVs suppressed the function of T cells by binding to PD-1 on T cells [3]. Here, we investigated whether PD-L1-positive I-sEVs could function similarly to PD-L1-positive T-sEVs regarding T cell inhibition. First, we used confocal microscopy to show a physical interaction between Jurkat T cells labelled with phalloidin (an F-actin cytoskeleton dye) and CFSE-labelled PD-L1-positive sEVs derived from tumor cells (CAL27 oral cancer and H1975 lung cancer cells) or stimulated immunocytes (Supplementary Fig. 4A and Fig. 4a). As expected, both CAL27 and H1975 cell-derived sEVs inhibited T cell proliferation and cytokine production, as demonstrated by the decreased Ki-67 and IFN-γ levels (Fig. 4b–d). However, sEVs derived from Raji or THP-1 cells failed to suppress T cell proliferation and IFN-γ secretion (Fig. 4b–d). Similarly, sEVs derived from primary T cells, macrophages and DCs did not suppress Jurkat T cell proliferation (Supplementary Fig. 4B). Then, we detected the downstream molecules of the PD-L1/PD-1 pathway in sEV-treated Jurkat T cells. T-sEVs, but not I-sEVs, significantly reduced phosphorylated ERK (p-ERK) and phosphorylated Akt (p-Akt) levels (Fig. 4e). Using a soluble Fc-tagged form of the extracellular region of PD-1 protein revealed that T-sEVs strongly bound PD-1, but I-sEVs did not (Fig. 4f). Thus, although I-sEVs express PD-L1 on their surfaces, they do not inhibit T cells, potentially resulting from the loss of PD-1 binding ability.
Fig. 4. PD-L1-positive I-sEVs did not suppress T cells in vitro.
a Confocal microscopy analysis of activated Jurkat T cells after incubation with CFSE-labeled T-sEVs (CAL27, H1975) or stimulated I-sEVs (Raji, THP-1). b Representative contour plots of Ki-67 expression in activated Jurkat T cells after treatment with sEVs. c The proportions of Ki-67-positive Jurkat T cells in (b). d IFN-γ released by activated Jurkat T cells after treatment with sEVs as measured by ELISA. e Western blot analysis of Akt, p-Akt, ERK and p-ERK levels in T cells treated with sEVs. All lanes were loaded with the same amount of total protein. f High-resolution flow cytometry of soluble PD-1-Fc protein binding to tumor cell- or immune cell-derived sEVs. The soluble PD-1 Fc protein was stained with Fc-PE immunofluorescent antibody. Data are mean ± SD of three independent experiments (c, d, f). P values are from one-way ANOVA test (c, d) and two-sided unpaired t test (f).
Co-expression of CD80 restricted the immunosuppressive function of sEV PD-L1
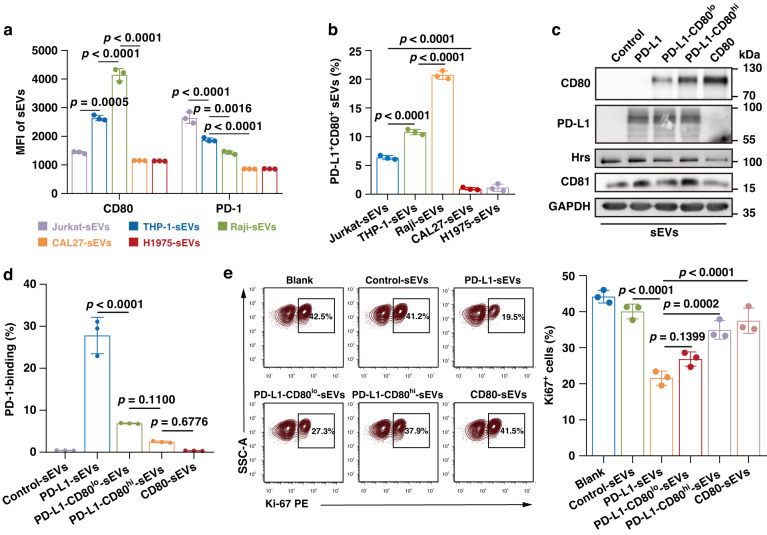
Recent evidence indicates that PD-L1 and its binding partners (e.g., CD80 and PD-1) can engage with each other through trans-PD-L1/CD80 or trans-PD-L1/PD-1 interactions between cells and cis-PD-L1/CD80 or cis-PD-L1/PD-1 on the same cell with a higher affinity [18, 19]. Importantly, the cis-structure restricts the formation of trans-PD-L1/PD-1 interactions between cells, thereby abrogating the inhibitory function of PD-L1 [13, 18]. Of interest, PD-L1 was widely present on both T-sEVs and I-sEVs, but CD80 and PD-1 were exclusively expressed on I-sEVs, as demonstrated by the results from high-resolution flow cytometry and western blot analysis (Fig. 5a, b and Supplementary Fig. 4C–G). Correspondingly, although I-sEVs scarcely bound PD-1 (Fig. 4f), they strongly bound PD-L1 compared to T-sEVs (Supplementary Fig. 4H), suggesting that I-sEVs may express PD-L1 binding partners.
Fig. 5. Co-expression of CD80 on sEVs disrupted PD-L1-positive sEV-mediated PD-1 binding and T cell inhibition.
a Quantification for the MFI of CD80 and PD-1 on sEVs derived from human cell lines, the MFI is tested by high-resolution flow cytometry. b The percentages of co-expressing CD80 and PD-L1 sEVs derived from human cell lines. c Western blot analysis of CD80 and PD-L1 on sEVs derived from CD80 and/or PD-L1-expressing 293E cells. All lanes were loaded with the same amount of total protein. d The proportions of PD-1-binding of sEVs treated with indicated sEVs. e Representative contour plots of T cells (stimulated with PHA) after incubation with indicated sEVs examined for the expression of Ki-67. The proportions of Ki-67 positive T cells are shown in the right panel. Data are mean ± SD of three independent experiments. P values are from one-way ANOVA test.
On the basis of the above findings, we speculated that co-expressed CD80 or PD-1 on PD-L1-positive sEVs may disrupt the trans-ligation of sEV PD-L1 to PD-1 on T cells, thereby repressing the immunosuppressive effects. To test this hypothesis, we constructed model sEVs by expressing the same level of exogenous PD-L1 but different levels of exogenous CD80 using 293E cells, which do not express endogenous PD-L1 or CD80 (Fig. 5c and Supplementary Fig. 5). The PD-1 binding assay revealed that sEVs derived from PD-L1-expressing 293E cells strongly bound PD-1, whereas the PD-1 binding ability was much weaker in sEVs co-expressing CD80 (Fig. 5d). Moreover, the ability of sEV PD-L1 in PD-1 binding was further decreased when the level of CD80 was increased (Fig. 5d), indicating that the PD-1 binding ability was dependent on the relative level of PD-L1 and CD80 on the sEVs. We then examined the effects of co-expression of CD80 on sEV PD-L1-mediated immunosuppression. As expected, sEVs isolated from PD-L1-expressing 293E cells inhibited the proliferation of T cells (Fig. 5e). However, the co-expression of CD80 on sEVs attenuated this inhibition, and the magnitude of attenuation was closely associated with the CD80 level (Fig. 5e). Similarly, the co-expression of PD-1 on sEVs also attenuated the inhibitory effect of sEV PD-L1 on T cell proliferation (Supplementary Fig. 6A, B). However, the magnitude of attenuation through PD-1 co-expression was lower than that caused by CD80 co-expression. Additionally, the co-expression of CD80 on tumor cells has been found to inhibit PD-L1-mediated T cell suppression [20]. We also overexpressed CD80 on tumor cells and collected sEVs (Supplementary Fig. 7A). The results revealed that the T-sEV PD-L1-mediated PD-1 binding and T cell inhibition were neutralized by the CD80 co-expression (Supplementary Fig. 7B, C). Overall, these findings in different cell lines demonstrated a general phenomenon of the inhibition of sEV PD-L1-induced immunosuppression when CD80 was co-expressed.
Co-expression of CD80 attenuated sEV PD-L1-induced tumor progression across different tumor types
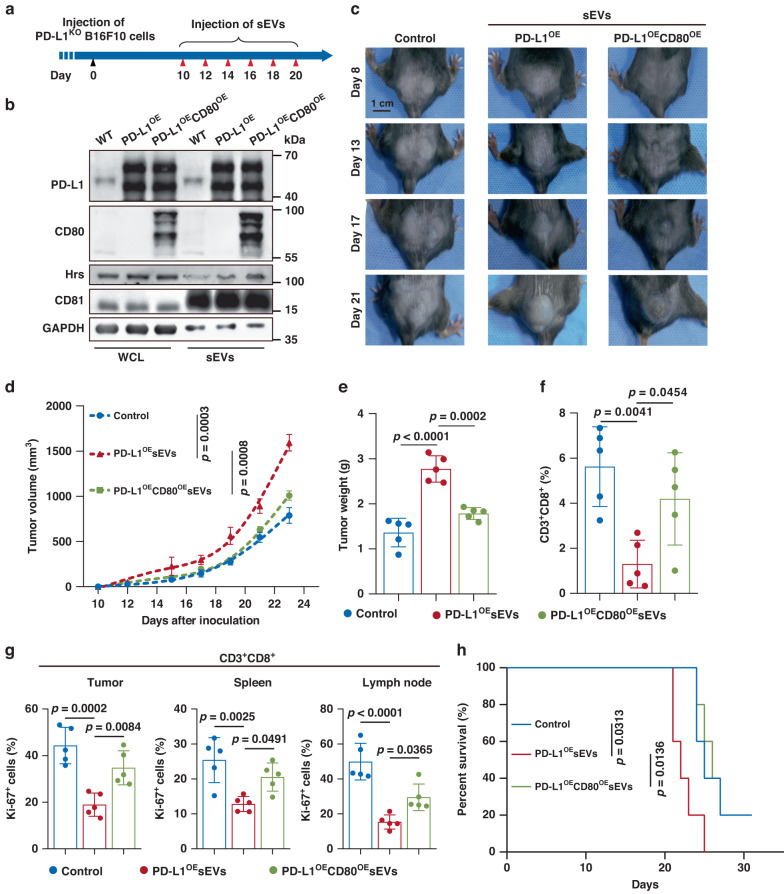
To further verify the above findings in vivo, we established a melanoma xenograft model in C57BL/6 mice using CRISPR/Cas9-mediated PD-L1 knockout B16F10 cells (PD-L1KO B16F10) (Supplementary Fig. 8A). The effect of sEV PD-L1 on tumor progression and its attenuation by CD80 co-expression were tested by injecting sEVs carrying different levels of PD-L1 and CD80 into mice bearing PD-L1KO B16F10 tumors (Fig. 6a, b). Tumor growth was significantly promoted by sEVs derived from PD-L1-overexpressing (PD-L1OE) B16F10 cells (Fig. 6c–e), but not those derived from PD-L1KO B16F10 cells (Supplementary Fig. 8B, C), confirming the tumor-promoting effects of sEV PD-L1. Additionally, unlike sEVs derived from PD-L1OE B16F10 cells, sEVs derived from B16F10 cells co-overexpressing PD-L1 and CD80 (PD-L1OECD80OE) showed no significant effect on tumor growth (Fig. 6c–e). The flow cytometry and immunofluorescence results further revealed that the number of tumor-infiltrating CD8+ T lymphocytes (TILs) was decreased significantly after the injection of PD-L1OE sEVs (Fig. 6f and Supplementary Fig. 8D). Additionally, PD-L1OE sEVs decreased the proportion of proliferating CD8+ T cells in the spleen and lymph nodes (Fig. 6g), suggesting that sEV PD-L1 systemically suppressed antitumor immunity. However, these significant inhibitory effects on TILs were not observed with PD-L1OECD80OE sEVs (Fig. 6f, g). Moreover, PD-L1OE sEVs injection significantly shortened the survival time of mice bearing PD-L1KO B16F10 tumors, while PD-L1OECD80OE sEVs showed no significant effect (Fig. 6h). These results suggest that CD80 co-expression prevented the immunosuppressive and tumor-promoting effects of sEV PD-L1.
Fig. 6. Co-expression of CD80 suppressed PD-L1-positive sEV-induced immunosuppression and tumor progression in melanoma model.
a Schematic of experimental design of tumor-bearing mice. b Western blot analysis of PD-L1 and CD80 in WCL and sEVs isolated from wild-type (WT), PD-L1OE and PD-L1OECD80OE B16F10 cells. GAPDH was set as the loading control for WCL, CD81 was set as the loading control for sEVs. All lanes were loaded with the same amount of total protein. The results shown represent three independent experiments. c Representative images showing the growth of PD-L1KO B16F10 tumors in C57BL/6 mice after indicated treatments. d Tumor growth curves of PD-L1KO B16F10 tumors with indicated treatments (n = 5 for each group). e Tumor weights of PD-L1KO B16F10 tumors in C57BL/6 mice after indicated treatments (n = 5 for each group). f The proportions of CD8+ TILs in PD-L1KO B16F10 tumors (n = 5 for each group). g The proportions of Ki-67+ CD8+ TILs or splenic or lymphatic CD8+ T cells after indicated treatments (n = 5 for each group). h Survival curves of PD-L1KO B16F10 tumors in C57BL/6 mice after indicated treatments (n = 5 for each group). Data are mean ± SD (d–g). P values are from two-way ANOVA (d), one-way ANOVA (e–g) or log-rank test (h).
Next, we asked whether CD80 on sEVs restricted sEV PD-L1-induced immunosuppression and tumor progression only in the melanoma model. Using mouse MC38 colorectal cancer model, the effects of sEVs derived from wild-type or CD80-overexpressing (CD80OE) MC38 cells were analyzed (Supplementary Fig. 9A). It was revealed that sEVs derived from wild-type MC38 cells obviously promoted the tumor growth, but sEVs derived from CD80OE MC38 cells exhibited no significant effect (Supplementary Fig. 9B–D). Consistently, sEVs from wild-type but not CD80OE MC38 cells induced significant suppression on TILs, splenic and lymphatic CD8+ T cells (Supplementary Fig. 9E) and decreased the lifespan of tumor-bearing mice (Supplementary Fig. 9F). These results together demonstrate that co-expression of CD80 would attenuate sEV PD-L1-induced tumor progression.
Discussion
Recently, increasing evidence has revealed the important roles of PD-L1-positive T-sEVs in suppressing antitumor immunity [2, 3, 21]. In the present study, we revealed that immunocytes, especially stimulated immunocytes, could also release PD-L1-positive sEVs, and PD-L1-positive I-sEVs were widely present in both HD and HNSCC patients. Although I-sEVs carried abundant PD-L1 on their surface, these vesicles did not exert significant immunosuppressive effects and differed from PD-L1-positive T-sEVs. Further investigations revealed that the co-expression of PD-L1 and its binding partners, PD-1 and CD80, on sEVs disrupted the binding of sEV PD-L1 to its receptor, PD-1, on T cells and attenuated sEV PD-L1-mediated immunosuppression in vitro and in vivo. These findings provide important information in that PD-L1-expressing sEVs may not exhibit immunosuppressive effects when they co-express a large amount of CD80 and/or PD-1.
Although PD-L1 and CD80 are well-known ligands of PD-1 and CD28/CTLA-4, respectively, previous studies have demonstrated that these molecules can also interact with each other on different immune cells in trans [22, 23]. Recent studies have also revealed that PD-L1 and CD80 interact in cis when expressed on the same immune cell [12, 13, 24]. The cis-structure can restrict the binding of PD-L1 to its receptor PD-1 on T cells, thereby blunting the immunosuppressive role of PD-L1. In the present study, we found that CD80 and PD-L1 were abundantly co-expressed on sEVs derived from stimulated immunocytes, however, negligible expression was observed in sEVs derived from tumor cells. Correspondingly, PD-L1 carried on sEVs derived from immune cell lines and primary immune cells enriched with CD80 did not inhibit T cell activation in vitro. Likewise, we overexpressed CD80 on tumor cells and collected sEVs carrying both CD80 and PD-L1. The inhibitory function of PD-L1-positive T-sEVs was neutralized by co-expression of CD80 in vitro. Additionally, we injected sEVs into the circulating blood of mice at the approximate physiological level of I-sEVs. The results confirmed the neutralizing function of CD80 in vivo. Collectively, these results suggest a general phenomenon in which the co-expression of CD80 could restrict the immunosuppression of sEV PD-L1 in both immune and non-immune cells. However, due to the small size and fragile property of sEVs and the current limitation in detection techniques, it would be difficult for us to verify whether PD-L1 indeed interacts with CD80 in cis on I-sEVs at present. Therefore, further investigations remain needed to dissect the precise mode of interaction between PD-L1 and CD80 on sEVs.
In addition to CD80, PD-1 can interact with PD-L1 in cis when expressed on the same cell and prevent PD-L1 from triggering T cell PD-1 [18]. In this study, the co-expression of PD-1 could also attenuate sEV PD-L1-mediated immunosuppression, although this effect seemed less significant than that with CD80. Notably, CD80 and PD-1 may be differentially expressed on different immunocytes and their derived sEVs in different settings (Fig. 5a and Supplementary Fig. 4D). This finding suggests that both CD80 and PD-1 would attenuate the function of PD-L1 on sEVs, but their contributions may differ in certain contexts. To simplify the working model, however, we focused on the effect of CD80 rather than PD-1 in the present study.
Although I-sEVs did not exert immunosuppressive effects on T cells due to the co-expression of CD80, they may still play important roles in immune responses in both the tumor microenvironment and draining lymph nodes [25, 26]. According to previous studies, sEVs from different immune cells may be a double-edged sword in immunity as they can either reshape a pro-inflammatory microenvironment to inhibit tumor progression, or promote tumor progression by inhibiting the killing effect of immune cells or promoting tumor and immunosuppressive immune cells [25, 26]. A recent study revealed that activated T cell-derived sEVs attenuated PD-L1-induced immune dysfunction in breast cancer [27]. However, Wang et al. demonstrated that functionally exhausted T cells could secrete numerous sEVs, which impaired T cell proliferation, activity and cytokine production [28]. Thus, similar to their donor cells, I-sEVs can either promote or inhibit tumor development. However, further research on I-sEVs is required to shed light on their precise roles.
Our results demonstrate the cellular origin and functional heterogeneity of PD-L1-positive sEVs, indicating the diverse roles of T-sEVs and I-sEVs in tumor microenvironment and clinical application. For I-sEVs co-expressed with CD80 and PD-L1, we demonstrate a lack of immunosuppression, but their precise roles still need to be explored in the future. It is possible that the CD80 co-expression on I-sEVs could prevent their parental cells and other immune cells from sEV PD-L1-induced dysfunction or apoptosis. Meanwhile, PD-L1-positive sEVs derived from tumor cells and immunocytes exhibit different clinical significance in HNSCC. Compared to PD-L1-positive I-sEVs, PD-L1-positive T-sEVs are more correlated with the diagnosis and prognosis of HNSCC. This suggests that PD-L1-positive sEVs, as a potential biomarker for predicting the outcomes of anti-PD-1 immunotherapy [3, 5, 6], should better be analyzed with cellular origins to improve their predictive performance in the future.
Overall, these findings suggest that the immunosuppressive effects of PD-L1-positive sEVs can be restricted by CD80 co-expression, demonstrating the important but previously unknown heterogeneity of PD-L1-positive sEVs in terms of cellular origins, molecular compositions, and biological functions. This study also provides the possible reasons behind the heterogeneity of PD-L1-positive sEVs, and offers a reliable rationale for the early and efficient prediction of immunotherapy response by specifically detecting PD-L1-positive T-sEVs before the treatment. However, a greater understanding of the mechanisms underlying the heterogeneity of PD-L1-positive sEVs and other important co-expressed molecules is required to further improve the prediction accuracy.
Supplementary information
Supplementary Figures and Supplementary Methods
Author contributions
GC conceived and designed the study. JYL, ZLZ, QYF and JBL performed the experiments. JYL, ZLY, MW and BL analyzed and interpreted the data. GC, JYL and ZLY wrote and edited the manuscript. All authors approved the final version of this manuscript.
Funding
This work was supported by the National Key R&D Program of China (2019YFA0210500), National Natural Science Foundation of China (82341023, 81922038, 81801842), Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018005), The Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZLCX20212300), The Fundamental Research Funds for the Central Universities (2042022dx0003).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of School and Hospital of Stomatology, Wuhan University (IRB no.2022.B05) and Zhongnan Hospital of Wuhan University (IRB no. 2021059 K). Participants gave informed consent to participate in the study before taking part. The permission for animals was approved by the Institutional Research Ethics Committee of School and Hospital of Stomatology, Wuhan University (IRB no. S07921060C).
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jin-Yuan Liu, Zi-Li Yu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02369-w.
References
- 1.Dong H, Strome S, Salomao D, Tamura H, Hirano F, Flies D, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 2.Poggio M, Hu T, Pai C, Chu B, Belair C, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–27.e413. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Huang A, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins P, Morelli A. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros M, Arnould L, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9:1710899. doi: 10.1080/20013078.2019.1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serratì S, Guida M, Di Fonte R, De Summa S, Strippoli S, Iacobazzi R, et al. Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol Cancer. 2022;21:20. doi: 10.1186/s12943-021-01490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timaner M, Kotsofruk R, Raviv Z, Magidey K, Shechter D, Kan T, et al. Microparticles from tumors exposed to radiation promote immune evasion in part by PD-L1. Oncogene. 2020;39:187–203. doi: 10.1038/s41388-019-0971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Soder R, Abhyankar S, Abdelhakim H, Braun M, Trinidad C, et al. WJMSC-derived small extracellular vesicle enhance T cell suppression through PD-L1. J Extracell Vesicles. 2021;10:e12067. doi: 10.1002/jev2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir M, Butte M, Freeman G, Sharpe A. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst R, Soria J, Kowanetz M, Fine G, Hamid O, Gordon M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodoraki M, Hoffmann T, Whiteside T. Separation of plasma-derived exosomes into CD3 and CD3 fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin Exp Immunol. 2018;192:271–83. doi: 10.1111/cei.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiura D, Maruhashi T, Okazaki I, Shimizu K, Maeda T, Takemoto T, et al. cisRestriction of PD-1 function by -PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364:558–66. doi: 10.1126/science.aav7062. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Lee C, Lin C, Gassen R, Xu X, Huang Z, et al. PD-L1:CD80 Cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51:1059–73.e1059. doi: 10.1016/j.immuni.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein J, Lipson E, Cottrell T, Forde P, Anders R, Cimino-Mathews A, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res. 2020;26:545–51. doi: 10.1158/1078-0432.CCR-19-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, et al. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano. 2018;12:671–80. doi: 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- 16.Cohen E. Role of epidermal growth factor receptor pathway-targeted therapy in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2659–65. doi: 10.1200/JCO.2005.05.4577. [DOI] [PubMed] [Google Scholar]
- 17.Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20. doi: 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Harrison D, Song Y, Ji J, Huang J, Hui E. Antigen-presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep. 2018;24:379–90.e376. doi: 10.1016/j.celrep.2018.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhri A, Xiao Y, Klee A, Wang X, Zhu B, Freeman G. In CisPD-L1 binds to B7-1 only on the same cell surface. Cancer Immunol Res. 2018;6:921–9. doi: 10.1158/2326-6066.CIR-17-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haile S, Bosch J, Agu N, Zeender A, Somasundaram P, Srivastava M, et al. Tumor cell programmed death ligand 1-mediated T cell suppression is overcome by coexpression of CD80. J Immunol. 2011;186:6822–9. doi: 10.4049/jimmunol.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Zhong W, Wang B, Yang J, Yang J, Yu Z, et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev Cell. 2022;57:329–43.e327. doi: 10.1016/j.devcel.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Flies D. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpe A, Pauken K. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura D, Okazaki I, Maeda T, Maruhashi T, Shimizu K, Arakaki R, et al. PD-1 agonism by anti-CD80 inhibits T cell activation and alleviates autoimmunity. Nat Immunol. 2022;23:399–410. doi: 10.1038/s41590-021-01125-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Shi Y. Exosomes derived from immune cells: the new role of tumor immune microenvironment and tumor therapy. Int J Nanomedicine. 2022;17:6527–50. doi: 10.2147/IJN.S388604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi S, Cho H, Yea K, Baek M. Immune cell-derived small extracellular vesicles in cancer treatment. BMB Rep. 2022;55:48–56. doi: 10.5483/BMBRep.2022.55.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu Y, Yang Y, Yang R, Liu C, Hsu J, Jiang Z, et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40:4992–5001. doi: 10.1038/s41388-021-01896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Shen H, He Q, Tian W, Xia A, Lu X. Exosomes derived from exhausted CD8 + T cells impaired the anticancer function of normal CD8 + T cells. J Med Genet. 2019;56:29–31. doi: 10.1136/jmedgenet-2018-105439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Supplementary Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.