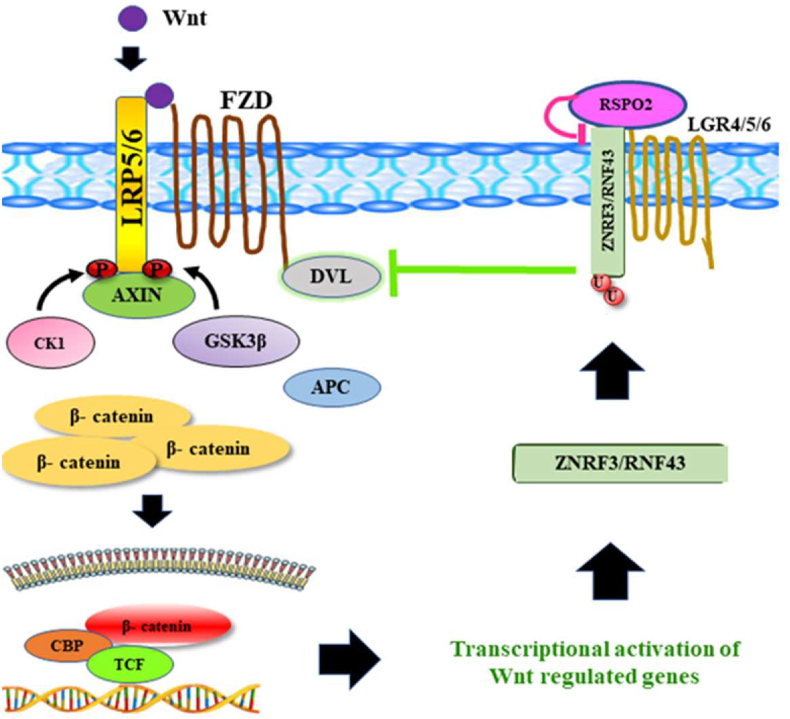
Figure 2.
This diagram represents a regular Wnt pathway occurring in normal cells. Activation of Wnt signaling is initiated by the binding of Wnt ligand to the Frizzled-LRP5/6 co-receptor complex, resulting in phosphorylation of Dishevelled (DVL) and LRP5/6, which in turn causes inhibition of GSK3β and further inactivation of the destruction complex AXIN, GSK3β, APC, and CK1. Collectively, Wnt ligand stimulation relocalizes the destruction complex to the plasma membrane where their inhibition remains controversial. Inactivating the destruction complex leads to the cytoplasmic stabilization of β-catenin, and thus, stabilized β-catenin moves to the nucleus and replaces the repressive transcription complex with the active transcription factors like TCF and CBP to induce the expression of Wnt-responsive target genes. However, Wnt signaling is regulated in a negative feedback manner through RSPO signaling. Expression of the ZNRF3/RNF43 degrades Frizzled receptors assisted by DVL protein through ubiquitination. As a part of feedback regulation, the antagonistic effect of ZNRF3/RNF43 on Wnt signaling is counteracted by binding of RSPO ligand to LGR4/5-ZNRF3/RNF43 to form multiple ligand-receptor complexes, inducing degradation and ubiquitination of ZNRF3/RNF43, which inhibits the degradation of Frizzled and achieves a high level of β-catenin. Hence, the level of β-catenin is regulated with the help of this cyclic loop.