Abstract
m6A methylation is the most frequent modification of mRNA in eukaryotes and plays a crucial role in cancer progression by regulating biological functions. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BP) are newly identified m6A ‘readers’. They belong to a family of RNA-binding proteins, which bind to the m6A sites on different RNA sequences and stabilize them to promote cancer progression. In this review, we summarize the mechanisms by which different upstream factors regulate IGF2BP in cancer. The current literature analyzed here reveals that the IGF2BP family proteins promote cancer cell proliferation, survival, and chemoresistance, inhibit apoptosis, and are also associated with cancer glycolysis, angiogenesis, and the immune response in the tumor microenvironment. Therefore, with the discovery of their role as ‘readers’ of m6A and the characteristic re-expression of IGF2BPs in cancers, it is important to elucidate their mechanism of action in the immunosuppressive tumor microenvironment. We also describe in detail the regulatory and interaction network of the IGF2BP family in downstream target RNAs and discuss their potential clinical applications as diagnostic and prognostic markers, as well as recent advances in IGF2BP biology and associated therapeutic value.
Keywords: Cancer, IGF2BP, Immunosuppressive TME, m6A, Molecular mechanism, Therapy
Introduction
Epigenetic mechanisms, mainly involving chromatin rearrangement, DNA methylation, RNA interference, RNA modification, and histone modification, refer to reversible and heritable phenotypes, which do not arise from altered DNA sequences.1 There are over 170 types of RNA modifications, including N6-methyladenosine (m6A), N6,2′-O-dimethyladenosine (m6Am), 5-methylcytidine (m5C), 5-hydroxylmethylcytidine (hm5 C), and N1-methyladenosine (m1A). Among these, m6A methylation is the most abundant internal mRNA modification in eukaryotes, and m6A sites are enriched in 3′ untranslated regions (UTR) especially located near stop codons.2 The methylation of m6A sites regulates the post-transcriptional modifications of RNAs in several ways, including splicing, exporting, stabilization, translation, and decay. Recently, it has been demonstrated that m6A can participate in the regulation of biological processes through a variety of mechanisms, thus playing a key role in cancer.3
The methylation of m6A is regulated by regulators, consisting of ‘writers’, ‘erasers’, and ‘readers’. Writers are methyltransferases, which install m6A methyl groups on RNA, while erasers are demethylases, which remove RNA m6A methylation reversibly. Readers are proteins that perform the biological functions of m6A methylation, and IGF2BPs are newly identified members of this group and include IGF2BP1, IGF2BP2, and IGF2BP3.4 However, as newly discovered m6A readers, the effects of IGF2BP in various malignancies and their associated mechanisms are still poorly understood.
Initially, researchers identified a protein with four K-homologous (KH) domains that is highly expressed in pancreatic cancer (PC) and named it KOC.5 With further research, the understanding of this protein grew, and was later named IGF2BP3. IGF2BP1, IGF2BP2, and IGF2BP3 belong to the family of RNA-binding proteins (RBPs), which participate in determining the fate of mRNAs transcripts by becoming a structural component of messenger ribonucleoprotein particles (mRNPs).6, 7, 8 Recently, a large number of studies have emerged investigating the function of IGF2BPs as m6A readers for recognizing m6A methylation. These studies provide evidence that IGF2BPs regulate gene expression by binding to the m6A binding sites of target mRNAs, thus intervening in various stages of RNA metabolism to influence many oncogenic processes, such as maintaining cancer stem cell stemness, promoting cancer proliferation, migration, glycolysis, cell cycle transition, and angiogenesis.4,9, 10, 11, 12, 13, 14 Convincing evidence that IGF2BP mediates tumor bio-behavioral changes and regulates the progression of various malignant diseases including pancreatic, hepatocellular, breast, and colorectal cancers through m6A methylation modifications.15, 16, 17 For example, IGF2BP2 promoted stem cell properties in breast cancer by stabilizing m6A-modified DROSHA mRNA.18 Herein, we propose that the role of IGF2BPs in the tumor microenvironment (TME) is multifactorial, not only promoting cancer biological properties but also their relationship with immune cells prompting us to further explore their role in the immunosuppressive TME.
The global incidence of malignant cancers is increasing and exploring their pathogenesis is of great significance for the early diagnosis and treatment of malignant cancers. Thus, it is particularly important to study the upstream regulatory mechanism of IGF2BPs. Understanding how these are expressed in malignant cancers will lead to a better understanding of the mechanisms responsible for tumorigenesis. In this review, we summarize various aspects involved in IGF2BP-mediated regulation of gene expression and the influence of different factors on their RNA binding capacity. Meanwhile, the specific mechanism of IGF2BPs in regulating RNA stability, localization, translation, and other processing in cancers is updated and the post-transcriptional regulatory network of its targeting noncoding RNAs (ncRNAs) is introduced. Finally, we discuss their potential value in cancer diagnosis, prognosis, and treatment, and track the development status of selective inhibitors of IGF2BPs to explore the possibility of their clinical application (Fig. 1).
Figure 1.
Upstream and downstream factors involving IGF2BPs in different cancer types. The upstream regulatory mechanisms of IGF2BPs include chromosomal rearrangements, epigenetic modifications, transcriptional and post-transcriptional control, post-translational modifications, and degradation regulation. IGF2BPs can regulate immunosuppressive TME through its effects on the RNA processing of mRNAs and ncRNAs. This process is influenced by many proteins, mRNAs, and ncRNAs.
The related structure of IGF2BPs protein for binding with the target RNAs
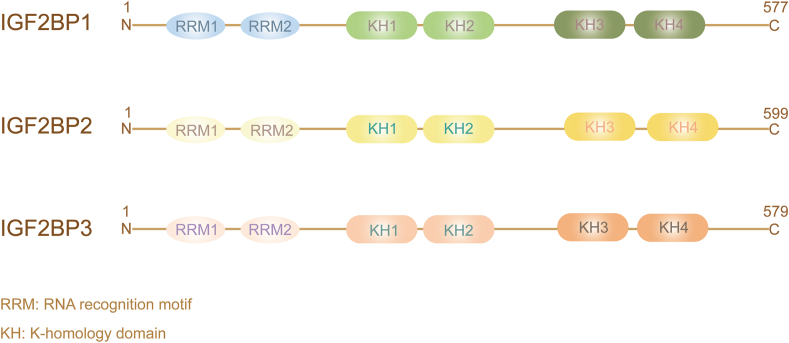
IGF2BPs are a family of insulin-like growth factor 2 mRNA-binding proteins that contain IGF2BP1–3. IGF2BPs' aliases include IMP1–3, VICKZ1–3 (based on the first letter of its founding members), CRD-BP (IGF2BP1), ZBP1 (IGF2BP1 homologue in chickens), Vg1RBP/Vera (homologue in Xenopus), or KOC (IGF2BP3),5 and are considered new m6A readers.4 However, it should be noted that Z-DNA binding protein 1 (ZBP1) should not be confused with IGF2BP1 and U3 small nucleolar ribonucleoprotein (IMP3) with IGF2BP3, as these share a common symbol/alias. IGF2BPs are highly conserved in many species, such as humans, chimpanzees, Rhesus monkeys, dogs, cows, rats, chickens, and zebrafish. IGF2BPs contain two RNA recognition motifs (RRM) at the N-terminal and four KH domains at the C-terminal19 (Fig. 2). From previous studies, we know that the four KH domains are essential for intracellular granule formation, trafficking of IGF2BP1, and RNA binding in vitro.20 Further research has revealed that the KH1/2 domain may be necessary for the stabilization of IGF2BP-RNA complexes,8 and the KH3/4 domain is essential for m6A recognition and binding with target RNAs.4,21 A recent study found that the high affinity of the KH1/2 domain for mRNA binding was achieved by interdomain coupling, which supported the role of IGF2BP1 recognition of known cancer targets.22 Different target RNAs require different KH domains for binding. For example, the KH1/2 domains of IGF2BP1 are necessary for binding to long ncRNA (lncRNA) KB-1980E6.3.9 Although circNSUN2 binds to the KH3-4 didomain of IGF2BP2,23 other studies have shown that all four KH domains of IGF2BP1 are essential for binding to KRAS or the microphthalmia-associated transcription factor (MITF) mRNA.24,25 Importantly, any single-point mutation in one of the four KH domains significantly affects the binding between IGF2BP1 and mRNA.26
Figure 2.
The structure of IGF2BPs. The three members of the IGF2BP family have similar structures: two RNA recognition motifs at the N-terminal and four K-homology domains at the C-terminal.
The regulation of IGF2BPs in cancers
The regulation process of IGF2BPs expression
IGF2BPs are expressed in the embryo and are also expressed in mature testicular germ cells and oocytes.27,28 In adult tissues, IGF2BPs except IGF2BP2 have low expression levels.29 IGF2BPs are increased or re-expressed in some malignant cancers and are therefore considered a conserved post-transcriptional enhancer of pro-oncogenic factors.30 The regulatory mechanisms of IGF2BP expression have not been fully elucidated, and therefore we will outline some regulatory modalities in cancers, including chromosomal rearrangements, epigenetic modifications, transcriptional control, post-translational modifications, degradation, and regulation.
Effect of chromosomal rearrangements on IGF2BPs expression
With the help of full transcriptome and whole genome sequencing, researchers have identified the chromosomal rearrangements occurring in thyroid cancer (TC), where the thyroid adenoma-associated (THADA) fusion to LOC389473 (the upstream region of the IGF2BP3 gene) and other regions in the vicinity increased the expression of full-length IGF2BP3 mRNA and protein.31 As we know, copy number alteration (CNA) is generally due to genomic rearrangements and is one of the mechanisms that lead to the overexpression of IGF2BP2 in hepatocellular carcinoma (HCC).32 However, CNAs are not the cause of the up-regulation of IGF2BP1 in anaplastic thyroid carcinomas (ATC),33 indicating that the expression regulation of IGF2BP1/2/3 in cancers is diverse.
Epigenetic modifications affect IGF2BPs expression
Under the action of DNA methyltransferase or histone deacetylase inhibitors, the expression of IGF2BP3 increased significantly in mouse osteosarcoma, indicating that the expression of IGF2BP3 is partly attributable to epigenetic regulation such as DNA demethylation and histone acetylation.34 Furthermore, researchers found that DNA methylation at the IGF2BP3 gene promoter contributed to its silencing in normal human tissues, in contrast to its almost complete demethylation in intrahepatic cholangiocarcinoma.35 In addition, histone acetylation on the promoter of IGF2BP1 promotes its transcriptional activation in melanoma cells.36 In pancreatic islet cancers, it was also verified that IGF2BP2 is epigenetically regulated by menin–histone methyltransferase complexes in a time-dependent manner.37 From this evidence, we know that one of the causes of IGF2BP re-expression in cancers is the alteration of epigenetic modifications.
Transcriptional and post-transcriptional control influence IGF2BPs expression
Exploring the regulation of gene transcription in the context of cancer helps to understand the occurrence and development of cancer. Some studies have identified the mechanism of IGF2BP transcriptional control in different cancers. In breast cancer, IGF2BP1 transactivation was shown to be associated with nuclear translocation of β-catenin, where a highly conserved element (CTTTG-TC) located near the IGF2BP1 promoter region was essential for IGF2BP1 transcriptional activity. Conversely, IGF2BP1 could also stabilize β-catenin mRNA, implying that there exists a positive feedback loop.38 There is also evidence that IGF2BP2 transcription was regulated by the high mobility group AT-hook 2 (HMGA2) and its tumor-specific truncated form HMGA2Tr. In detail, HMGA2 binds directly to the AT-rich regulatory region located in the first intron of IGF2BP2, while a consensus binding site for NF-κB adjacent to the AT-rich regulatory region can bind to NF-κB. Ultimately, they synergistically promote the transcription of IGF2BP2 in human liposarcomas.39 Nonetheless, MYC, a widely recognized oncogene, is not only a target mRNA for IGF2BP3 but it also effectively binds to the IGF2BP3 promoter in nasopharyngeal carcinoma (NPC) and increases its transcriptional activity, indicating that there may be a positive feedback loop between IGF2BP3 and MYC.4,40 In other cancers, such as triple-negative breast cancer (TNBC)41 and lung cancer,42 studies have explored their transcriptional regulation. Moreover, the positive feedback loops described above partially explain why IGF2BPs are highly expressed in malignant cancers, as these positive feedbacks may initiate cascade reactions that amplify their effects.
It has been shown that miRNAs can bind to IGF2BP to inhibit their expression.43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 In contrast, in colorectal cancer (CRC), the short 3′UTR transcript of IGF2BP1 increases IGF2BP1 protein expression as it lacks miRNA sites.65 Therefore, it can be speculated that competitive binding to miRNAs can also up-regulate IGF2BPs in cancers, and recent studies have confirmed this hypothesis.66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 Interestingly, IGF2BP3 stabilizes the lncRNA KCNMB2-AS1 in an m6A methylation-dependent manner, while KCNMB2-AS1 sponges miR-130b-5p and miR-4294 can induce the up-regulation of IGF2BP3,77 which then forms a positive feedback loop in cervical cancer. The mRNA of IGF2BP1 or IGF2BP3 is stabilized by the lncRNA THOR or DARS-AS1 in cancer cells.78,79 Therefore, the mechanisms by which ncRNAs regulate IGF2BP protein expression involve regulating gene transcription.
The influence of post-translational modifications on IGF2BPs
In post-translation and co-translation, mTOR can dually phosphorylate the Ser162 and Ser164 residue of IGF2BP2 located in the N-terminal linker region between RRM2 and KH1 in a rapamycin-sensitive manner.80,81 IGF2BP3 was also phosphorylated by mTOR at the Ser183 residue, and similarly, IGF2BP1 is co-translationally phosphorylated at Ser181 by mTOR in the mTOR complex 2 (mTORC2), and subsequently released from mTOR to bind target mRNAs, which protected the phosphorylation site from cellular protein phosphatases.82 Human embryonal rhabdomyosarcoma cells and mouse embryonic fibroblasts were used in these studies to demonstrate that phosphorylated IGF2BP could bind to downstream target RNAs, providing evidence for their role in cancer-promoting activity.
Regulation of IGF2BPs protein degradation
Regarding the degradation of IGF2BPs, researchers have recently reported that makorin ring finger protein 2 (MKRN2), an E3 ligase, acts as a cancer suppressor in neuroblastoma by mediating the ubiquitination of IGF2BP3.83 Ubiquitin-specific protease 11 (USP11), a deubiquitinating enzyme, prevented the ubiquitination and degradation of IGF2BP3 in colorectal cancer.84 Furthermore, SUMO1-mediated SUMOylation, a modification that regulates substrate protein expression and activity, prevents IGF2BP2 degradation in glioma through the ubiquitin proteasome pathway.85
ncRNAs can also regulate IGF2BP degradation in different ways. For example, circNDUFB2 is down-regulated in non-small cell lung cancer (NSCLC), but its overexpression improves ubiquitination and degradation of IGF2BP1/2/3 meditated by TRIM25, a member of the tripartite motif (TRIM) family of E3 ubiquitin ligases.86 LncRNA LINRIS prevented the degradation of IGF2BP2 via the autophagy-lysosome pathway in CRC by binding to the ubiquitination site K139 of IGF2BP2 in the absence of inhibition factors, such as GATA3.87 circNEIL3 could inhibit HECTD4-mediated degradation of IGF2BP3 via the ubiquitin proteasome in glioma.88 The differential regulation of IGF2BP degradation by ncRNAs also confirms that cancers have complex environments and regulatory mechanisms. In summary, preventing the degradation of IGF2BPs is another way to maintain their expression in cancers. In recent years, the development of molecules that specifically degrade targeted proteins is an emerging cancer treatment strategy; therefore, drugs targeting IGF2BP degradation may be a viable therapeutic approach.
Various factors affecting the binding ability of IGF2BPs and RNA
IGF2BPs are required to bind to different RNAs to influence cancer progression; therefore, the RNA binding ability of IGF2BPs is very important and can be affected by many factors, including proteins, mRNAs, and ncRNAs. For instance, the Aurora kinase A (AURKA) oncogene enhanced the IGF2BP2 binding to m6A methylation-modified transcripts in breast cancer, rather than promoting its nuclear translocation.18 Although lncRNAs belong to ncRNAs, recently researchers have found that some lncRNAs can also encode proteins. Zhu et al89 confirmed in different cancer cell lines that LINC00266-1 encodes a peptide called RNA-binding regulatory peptide (RBRP), which binds to the KH3-4 domain of IGF2BP and promotes the ability of IGF2BP1 to recognize the m6A sites on c-MYC mRNA. YBX1 is important in promoting the recognition of m6A-modified RNAs by IGF2BP1/2/3 in myeloid leukemia.90 Conversely, in Ewing sarcoma, ABCF1 mRNA acts as a sponge to bind to IGF2BP3 and limits its interaction with oncogenic target transcripts.91 Tyrosine protein phosphatase non-receptor type 13 (PTPN13) also inhibits the binding of IGF2BP1 to c-MYC to inhibit HCC cell proliferation and tumorigenesis.92 Although most ncRNAs are not translated into proteins, they have made great contributions to the regulation of genes, especially in the occurrence and development of cancers. Its regulatory effect on IGF2BPs could also manifest itself by affecting the binding ability of IGF2BPs and target RNAs. As shown in Table 1, some ncRNAs could promote binding processes to promote cancer progression,93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108 and some other ncRNAs could competitively bind to the KH domain of IGF2BP, thus interfering with the recognition of m6A modified RNAs by IGF2BP and inhibiting their expression to suppress cancers.109, 110, 111, 112, 113, 114 The above results suggest that the effects of ncRNAs on the binding capacity of IGF2BPs are complex. Therefore, it is very interesting to investigate this mechanism in depth, which will help us to better understand the role of IGF2BPs in cancers and their mechanisms, and to find the molecules that can specifically inhibit the binding ability of IGF2BP with RNAs to treat cancers more effectively.
Table 1.
Upstream regulators of IGF2BPs in cancers.
| Upstream regulators | IGF2BPs | Regulatory mechanisms of upstream factors on IGF2BPs | Effects on IGF2BPs expression | References |
|---|---|---|---|---|
| miR-98–5p, miR-625, miR-196b, miR-494, miR-21, miR-372, miR-708, miR-506, miR-873 | 1 | Inhibit IGF2BP1 expression by binding with its 3′UTR | ↓ | 43,44,46,53,55,56,58, 59, 60 |
| miR-485–5p, miR-216b, miR-141, miR-188, miR-138 | 2 | Inhibit IGF2BP2 expression by binding with its 3′UTR | ↓ | 45,52,54,61,62 |
| miR-34a, miR-129-1, miR-486 | 3 | Inhibit IGF2BP3 expression by binding to its 3′UTR | ↓ | 48,49,51 |
| let-7 family | 1/2/3 | Bind to the 3′UTR | ↓ | 57,63,64,232 |
| miR-200a | 2/3 | Target the 3′UTR of IGF2BP2/3 mRNA to down-regulate its expression | ↓ | 47 |
| miR-1275 | 1/2/3 | Inhibit IGF2BP1/2/3 expression by directly binding to their 3′UTR | ↓ | 50 |
| lncRNA THOR | 1 | Promote the mRNA stabilization activities of IGF2BP1 | ↑ | 79 |
| linc01134 | 1 | Function as ceRNA to up-regulate IGF2BP1 via sponging miR-324–5p | ↑ | 71 |
| lncRNA TRPM2-AS | 1 | Act as a microRNA sponge of miR-612 to up-regulate IGF2BP1 | ↑ | 72 |
| lncRNA PCAT6 | 1 | Function as ceRNA to up-regulate IGF2BP1 via sponging miR-513 | ↑ | 75 |
| circBICD2 | 1 | Regulate IGF2BP1 via miR-149–5p | ↑ | 69 |
| lncRNA MALAT1 | 2 | Competitively binding to miR-204 to up-regulate IGF2BP2 | ↑ | 66 |
| lncRNA RHPN1-AS1 | 2 | Act as a sponge for miR-596 to up-regulate IGF2BP2 | ↑ | 76 |
| circCD44 | 2 | Function as a sponge for miR-502–5p to up-regulate IGF2BP2 | ↑ | 73 |
| HBV-pgRNA | 3 | Function as a sponge for miR-let-7e-5p to up-regulate IGF2BP3 | ↑ | 67 |
| LINC00460 | 3 | Function as a sponge for miR-320b to up-regulate IGF2BP3 | ↑ | 70 |
| lncRNA KCNMB2-AS1 | 3 | Function as a sponge for miR-130b-5p and miR-4294 to up-regulate IGF2BP3 | ↑ | 77 |
| circIGHG | 3 | Bind to miR-142–5p and consequently elevated IGF2BP3 activity | ↑ | 68 |
| circHIPK3 | 3 | Promote IGF2BP3 expression via interacting with miR-654 | ↑ | 74 |
| menin-histone methyltransferases | 2 | In a time-dependent manner | ↑ | 37 |
| β-catenin | 1 | The nuclear translocation of β-catenin promotes IGF2BP1 transactivation | ↑ | 38 |
| HMGA2 and HMGA2Tr | 2 | Promote the transcription of IGF2BP2 together with NF-κB | ↑ | 39 |
| HNF4G | 2 | Bind to the promoter region of IGF2BP2 | ↑ | 42 |
| MYC | 3 | Bind to the promoter of IGF2BP3 and increase its transcriptional activity | ↑ | 40 |
| lncRNA DARS-AS1 | 3 | Stabilize the mRNA of IGF2BP3 | ↑ | 78 |
| mTOR | 1/2/3 | Phosphorylate IGF2BPs to promote their binding to downstream target mRNA | \ | 80, 81, 82 |
| lncRNA LINRIS, SUMO1 | 2 | Prevent IGF2BP2 degradation | ↑ | 85,87 |
| circNEIL3, USP11 | 3 | Prevent ubiquitination and degradation of IGF2BP3 | ↑ | 84,88 |
| MKRN2 | 3 | Mediate the ubiquitination of IGF2BP3 | ↓ | 83 |
| circNDUFB2 | 1/2/3 | Overexpression of CIRCNDUFB2 promotes the ubiquitination and degradation of IGF2BPs | ↓ | 86 |
| estrogen receptor-β | 3 | Inhibit IGF2BP3 expression by repressing EGFR | ↓ | 41 |
| YBX1 | 1/2/3 | Important for promoting the recognition of the m6A-modified RNAs | \ | 90 |
| RBRP, lncRNA-GHET1, lncRNA NEAT1, circXOP1 | 1 | Promote the recognition and interaction with target mRNA | \ | 89,97,99,101 |
| AURKA, lncRNA SNHG12, linc01305, lncRNA PCAT6, LINC01559, LINC00460, circITGB6, circNSUN2, circARHGAP29, circARHGAP12 | 2 | Strengthen IGF2BP2 interaction with target mRNA | \ | 18,23,93,94,96,100,104, 105, 106, 107 |
| HuR, BAG3, circ-0039,411, lncRNA DMDRMR, lncRNA CERS6-AS1, LINC00467, linc01305, hsa_circ_0003258 | 3 | Strengthen IGF2BP3 interaction with target mRNA | \ | 95,98,102,103,106,108,184,233 |
| PTPN13, lncRNA FGF13-AS1, lncRNA NBAT1, LINC00261, circPTPRA | 1 | Inhibit the interaction between IGF2BP1 and c-MYC mRNA | \ | 92,109,110,113,114 |
| LINC01093 | 1 | Inhibit the interaction between IGF2BP1 and GLI1 mRNA | \ | 112 |
| circ-TNPO3 | 3 | Act as a protein decoy for IGF2BP3 to regulate the MYC-SNAIL axis | \ | 111 |
| ABCF1 mRNA | 3 | Act as a sponge to limit the interaction with ABCG2, MMP9, and CD44 | \ | 91 |
IGF2BPs-induced regulation of cancer
IGF2BPs regulate cancer progression by affecting RNAs
Despite being RNA-binding proteins with similar sequence homology, IGF2BP1, IGF2BP2, and IGF2BP3, the proteins exhibit different RNA-binding properties and may be related to different target transcripts. Each family member regulates a unique pool of RNAs.115 Thousands of transcripts have been identified as targets of each IGF2BP protein, but the exact molecular mechanism by which IGF2BPs control these transcripts and participate in biological processes has just begun to be elucidated. The most recent research indicates that the oncogenic function of IGF2BPs may depend on its role as m6A readers.4 Therefore, in this section, we review previous studies, combined with recent novel insights, to summarize the mechanism of action of IGF2BPs in the regulation of mRNA processing (Table 2) and the regulatory network of IGF2BPs and ncRNAs in cancers.
Table 2.
Different mechanisms in regulating mRNA processes by IGF2BPs in cancers.
| IGF2BPs | Cancer | Target mRNA | Mechanisms of IGF2BPs in RNA processes | Roles of mRNA in cancers | References |
|---|---|---|---|---|---|
| 1 | Breast cancer | KRT7 | Serve as an m6A reader to promote mRNA stabilization | Promote breast cancer lunger metastasis | 10 |
| 1 | Lung adenocarcinoma | CTNNB1 | Promote mRNA stabilization | Oncogene | 101 |
| 1 | GC | SEC62 | Serve as an m6A reader to promote mRNA stabilization | Promote GC proliferation and inhibit cell apoptosis | 234 |
| 1 | CRC | β-TrCP1 | Prevent miRNA-dependent degradation | Serve as substrate recognition subunit of E3 ubiquitin ligase | 120 |
| 1 | CRC | LDHA | Bind to the 3′UTR of LDHA mRNA | Promote glycolysis | 171 |
| 1 | HCC | YES1 | Serve as an m6A reader to promote mRNA stabilization | Oncogene | 15 |
| 1 | HCC | LYPD1 | Stabilize mRNA in an m6A-dependent manner | Promote tumorigenesis | 122 |
| 1 | HCC | MKI67 | Promote mRNA stabilization | Promote cell proliferation and inhibit cell apoptosis | 235 |
| 1 | HCC | GLI1 | Promote mRNA stabilization | Promote cell proliferation and metastasis | 112 |
| 1 | HCC, ovarian cancer, lung cancer | SRF | Serve as an m6A reader and impair miRNA-dependent degradation | Enhance expression of genes that promote aggressive cancer phenotype | 11 |
| 1 | HCC, PC, ovarian cancer, melanoma, lung cancer, | E2F | Rely on 3′UTR-, miRNA-, and m6A-dependent to stabilize it | Promote G1/S cell cycle transition | 13 |
| 1 | Melanoma, ovarian cancer | eEF2 | \ | Enhance basal proliferation rates | 223 |
| 1 | Ovarian cancer | MDR1 | Promote mRNA stabilization | Regulate chemoresistance | 64 |
| 1 | Ovarian cancer | SIRT1 | Prevent miRNA-dependent degradation | Promote anoikis-resistance | 118 |
| 1 | Ovarian clear cell carcinoma | let-7 target mRNA (IGF2BP1, HMGA2, LIN28B) | Sequester mRNA into mRNP that do not contain RISC to stabilize it | Promote cancer cell growth and self-renewal | 116 |
| 1 | Endometrial cancer | SOX2 | Serve as an m6A reader to promote mRNA stabilization | An oncogenic transcriptional factor | 153 |
| 1 | Endometrial cancer | PEG10 | Serve as an m6A reader to promote mRNA stabilization | Promote cell cycle and cancer progression | 159 |
| 1 | Choriocarcinoma | RSK2, PPME1 | Promote mRNA stabilization | Promote cell migration and invasion | 236 |
| 1 | Osteosarcoma | c-MYC | Sequester mRNA into mRNP that do not contain RISC to stabilize it | Promote cancer cell proliferation | 7 |
| 1 | Seminoma | TFAP2C | Serve as an m6A reader to promote mRNA stabilization | Increase resistance to cisplatin | 163 |
| 1 | Breast cancer | E-cadherin, β-actin, α-actinin, Arp2/3 | Promote mRNA localization | Stabilize cell–cell connections and focal adhesions | 128 |
| 1 | Osteosarcoma, ovarian cancer | PTEN | Bind to a rare codon-comprising fragment of the PTEN ORF to stabilize it | Modulate cell polarization | 134 |
| 1 | Osteosarcoma, ovarian cancer, tumor-derived cells | MAPK4 | Inhibit mRNA translation through binding to MAPK4 3′UTR | Promote cell adhesion and migration | 134,237 |
| 1 | Rhabdomyosarcomas | cIAP1 | Promote mRNA translation | Mediate apoptotic resistance | 130 |
| 1 | HCC | HCV | Promote mRNA translation | \ | 131 |
| 1 | OSCC | BMI1 | Promote mRNA translation in an m6A-dependent manner | Promote cell proliferation and metastasis | 12 |
| 1/2/3 | cervical cancer, HCC | MYC | Serve as an m6A reader to promote mRNA stabilization | Promote cancer proliferation, migration, and invasion | 4 |
| 2 | HNSCC | SLUG | Serve as an m6A reader to promote mRNA stabilization | Lymphatic metastasis and EMT | 238 |
| 2 | PTC | APOE | Serve as an m6A reader to promote mRNA stabilization | Mediate glycolysis | 183 |
| 2 | Radioiodine-refractory papillary thyroid cancer | RUNX2 | Serve as an m6A reader to promote mRNA stabilization | Block the differentiation of radioiodine-refractory papillary thyroid cancer | 239 |
| 2 | Breast cancer | DROSHA | Serve as an m6A reader to promote mRNA stabilization | Maintain breast cancer stem-like cell stemness | 18 |
| 2/3 | LUAD | VANGL1 | Serve as an m6A reader to promote mRNA stabilization | Mitigate the effects of radiation on LUAD by increasing genes about DNA repair after the damage | 167 |
| 2 | Lung cancer | TK1 | Serve as an m6A reader to promote mRNA stabilization | Promote angiogenesis | 42 |
| 2 | GC | ZEB1 | LINC01559 recruits IGF2BP2 to stabilize ZEB1 mRNA | Serve as a transcription factor to combine with LINC01559, and promote cell proliferation, migration, and EMT | 94 |
| 2 | HCC | FEN1 | Serve as an m6A reader to promote mRNA stabilization | Promote HCC growth | 32 |
| 2 | PDAC | GLUT1 | Bind to the middle of GLUT1 mRNA and promote mRNA stabilization | Promote glycolysis and proliferation | 182 |
| 2 | CRC | KLF4 | Serve as an m6A reader to promote mRNA stabilization | A cancer suppressor gene; regulates intestinal epithelial homeostasis | 240 |
| 2 | CRC | SOX2 | Serve as an m6A reader to promote mRNA stabilization | Promote stemness features and metastatic | 152 |
| 2 | CRC | MTA1 | Serve as an m6A reader to promote mRNA stabilization | Promote cancer migration and invasion | 181 |
| 2 | CRC | RAF-1 | Prevent miRNA-dependent degradation | Promote cancer proliferation and survival | 121 |
| 2 | CRC | HK2 | Bind to the 5′UTR/3′UTR of HK2 mRNA as an m6A reader to promote mRNA stabilization | Promote glycolysis and proliferation | 185 |
| 2 | CRC | MSX1, JARID2 | LINC021 enhances its role as an m6A reader to promote mRNA stabilization | Promote tumorigenesis | 241 |
| 2 | Ovarian cancer | FGF9 | Promote mRNA stabilization | Induce chemoresistance and the polarization of TAMs toward the M2 phenotype | 104 |
| 2 | Prostate cancer | IGF1r | The mRNA stabilization may be related to m6A modification | Promotes bone metastasis and cancer growth | 96 |
| 2 | Prostate cancer | LDHA | Bind to the 3′UTR of LDHA mRNA to promote mRNA stabilization | Promote glycolysis | 93 |
| 2 | Malignant embryonic rhabdoid tumor, cervical cancer, breast cancer, HCC, CRC, lung cancer | HMGA1 | Bind to the 3′UTR of HMGA1 mRNA to promote mRNA stabilization and may be related to m6A modification | An oncogene; promote cancer proliferation, migration, and invasion | 81,105 |
| 2 | Glioblastoma | let-7 target mRNA (IGF2BP3, HMGA1, HMGA2, CCND1) | Prevent miRNA-dependent degradation | Preserve glioblastoma stem cell | 119 |
| 2 | Glioblastoma | CI/CIV | Promote the mRNA activity | Regulate OXPHOS | 137 |
| 2 | embryonic rhabdomyosarcoma | NRAS | Promote mRNA stabilization | oncogene | 242 |
| 2 | Embryonic rhabdomyosarcoma | IGF2 | Promote mRNA translation | \ | 80 |
| 2 | Radioiodine-refractory papillary thyroid cancer | ERBB2 | Increase translation efficacy through binding to the m6A methylation site | Contribute to acquired resistance to TKI | 133 |
| 3 | NPC | KPNA2 | Serve as an m6A reader to promote mRNA stabilization | Promote proliferation and metastasis | 40 |
| 1/3 | Cervical cancer | CD44 | Stabilize the 5.0 kb CD44 mRNA | Promote invadopodia formation | 129 |
| 3 | Breast cancer | CD44 | Regulate CD44 promoter activity | Promote cell proliferation, maintain stemness, and induce chemotherapy resistance | 179 |
| 3 | Breast cancer | PD-L1 | Serve as an m6A reader to promote mRNA stabilization | Inhibit cancer immune surveillance | 174 |
| 3 | Breast cancer | IGF2, MMP9, CD164 | Bind to the mRNA and promote mRNA stabilization | Involve in migration and invasion | 41 |
| 3 | Breast cancer | CERS6 | Promote mRNA stabilization | Promote cell proliferation, suppress cell apoptosis | 102 |
| 3 | Breast cancer | ABCG2 | Bind to ABCG2 mRNA and regulate its expression | Promote cell invasion | 243 |
| 3 | Esophageal cancer | KIF18A | Stabilize KIF18A mRNA | Promote cancer proliferation and migration and is associated with radioresistance | 244 |
| 3 | GC | HDGF | Serve as an m6A reader to promote mRNA stabilization | Secrete HDGF promotes cancer angiogenesis; nuclear HDGF increases glycolysis | 14 |
| 3 | GC | HIF1A | Serve as an m6A reader to promote mRNA stabilization | Promote cell migration and angiogenesis | 180 |
| 3 | HCC | pgRNA | Promote mRNA stabilization in an m6A-independent manner | Promote HCC proliferation, stemness, and tumorigenicity | 67 |
| 3 | HCC | TRAF5 | Promote mRNA stabilization | Promote cell proliferation and metastasis | 103 |
| 3 | CRC | KRAS, MAP2K1, TPR, CCNH | Promote mRNA stabilization with the help of ELAVL1 | Promote cancer proliferation | 245 |
| 3 | CRC | ABCB1 | Serve as an m6A reader to promote mRNA stabilization | Promote cancer chemoresistance | 246 |
| 3 | CRC | CCND1 | Serve as an m6A reader to promote mRNA stabilization | Promote cell cycle | 160 |
| 3 | CRC | VEGF | Serve as an m6A reader to promote mRNA stabilization | Promote angiogenesis | 160 |
| 2/3 | CRC | GLUT1 | Bind to 3′UTR of GLUT1 mRNA as an m6A reader | Promote glycolysis and proliferation | 185 |
| 3 | PDAC | HK2 | Promote mRNA stabilization | Participate glycolysis | 184 |
| 3 | Hematopoietic progenitor cell | CDK6, MYC | Stabilize the mRNA and/or enhance translation | Oncogene | 247 |
| 3 | ccRCC | CDK4 | Serve as an m6A reader to promote mRNA stabilization | Is associated with the G1/S transition | 95 |
| 3 | Cervical cancer, HCC | PDK4 | Serve as an m6A reader to promote mRNA stabilization | Promote glycolysis and ATP generation | 186 |
| 3 | Acute myeloid leukemia | COX2 | Bind to the 3′UTR of mRNA to promote mRNA stabilization | Inhibit cell apoptosis | 233 |
| 1/3 | Myeloid leukemia | MYC, BCL2 | Promote stability of m6A-modified mRNA with the help of YBX1 | Maintain the myeloid leukemia cell survival | 90 |
| 3 | AML | RCC2 | Serve as an m6A reader to promote mRNA stabilization | Promote AML progression | 248 |
| 3 | Human cancer cells | CCND1, CCND3, CCNG1 | Cooperate with HNRNPM to regulate post-transcriptional expression | Accelerate cell proliferation | 136 |
| 3 | Ewing sarcoma | CD164 | Bind to the mRNA to regulate its expression | Promote cancer invasion | 249 |
| 3 | Some kinds of solid cancers and fibrosarcoma | HMGA2, LIN28B | Sequester mRNA into mRNP that did not contain RISC | Oncogene | 117 |
| 3 | PDAC | 164 direct targe mRNA contained HMGA2, ZFP36L1, DCBLD2, CLDN1, CD44, ANTRX1, CLDN1, OLR1 | Bind to Ago2 (a RISC component) and act as a bi-modal regulator of mRNA stability | Promote cell migration, proliferation, and remodel focal adhesion | 123 |
| 3 | HCC | ZO-1 | Enhance Ago2-mRNA interactions to inhibit ZO-1 expression | Down-regulation of ZO-1 promotes cancer metastasis and invasion | 250 |
| 3 | LUAD | EIF4E-BP2 | Serve as an RNA-destabilizing factor to promote EIF4E-BP2 mRNA degradation | Promote EIF4E-mediated translation activation | 125 |
| 3 | CRC | ULBP2 | Bind to the 3′UTR of ULBP2 mRNA to destabilize it | Enable immune cells to recognize and destroy cells that express it | 124 |
| 2/3 | TNBC | PR | Recruit CNOT1 to destabilize PR mRNA | Promote the metastasis | 47 |
AML: acute myeloid leukemia; CRC: colorectal cancer; GC: gastric cancer; HCC: hepatocellular carcinoma; HNSCC: head and neck squamous cell carcinoma; LUAD: lung adenocarcinoma; NPC: nasopharyngeal carcinoma; OSCC, oral squamous cell carcinoma; PC, pancreatic cancer; PDAC: pancreatic ductal adenocarcinoma; PTC: papillary thyroid cancer; TNBC: triple-negative breast cancer.
Modulation of mRNA by IGF2BPs in cancers
IGF2BPs could increase the stability of mRNA
Since the discovery of the IGF2BP protein family, its role as RBPs has been continuously updated. IGF2BP proteins can influence RNA stability through a variety of mechanisms in different cancers, including sequestering mRNAs in mRNP that did not contain the RNA-induced silencing complex (RISC),7,116,117 preventing miRNA-dependent degradation,118, 119, 120, 121 and functioning as m6A readers that bind to m6A sites in RNA.4,11,18,122 Specifically, Huang et al4 identified three IGF2BPs that could preferentially bind to the “UGGAC” consensus sequence containing the “GGAC” m6A core motif and found that binding of IGF2BPs to the m6A methylation modification site in MYC mRNA could increase the stability of MYC mRNA as well as the translation efficiency in HCC and cervical cancer. Additionally, AURKA improved IGF2BP2 binding to the m6A-modified RNase III DROSHA transcript to stabilize DROSHA mRNA, which was further strengthened by binding of AURKA and the DROSHA transcript, thus promoting stem cell properties in breast cancer.18 Interestingly, in a recent study, Muller et al11 verified that IGF2BP1 stabilized SRF mRNA in cancer by weakening miRNA-dependent decay and binding to its m6A sites. We know that ALKBH5 is an m6A demethylase, and its down-regulation increases the m6A methylation level of oncogene LY6/PLAUR domain containing 1 (LYPD1), which was recognized to be stabilized by IGF2BP1 and thus promoted HCC oncogenesis.122
IGF2BPs can also destabilize RNAs. Ennajdaoui et al123 found that in addition to stabilizing mRNAs by competing with miRNAs for common binding sites in target mRNAs, IGF2BP3 promoted mRNA binding to argonaute 2 (Ago2) (a RISC-component) and was believed to be a bimodal regulator of mRNA stability in pancreatic ductal adenocarcinoma (PDAC). However, it is not difficult to determine that although IGF2BPs have opposite effects on the regulation of target RNA stability, they play an oncogenic role in cancers. For example, by destabilizing stress-induced ligands ULBP2 they promote cancer immune escape,124 or they destabilize eukaryotic translation initiation factor 4E binding protein 2 (EIF4E-BP2) to activate EIF4E, or they recruit CCR4-NOT transcription complex subunit 1 (CNOT1) to destabilize progesterone receptor (PR) mRNA, and these processes mediated by IGF2BP eventually promote cancer cell progression.47,125
IGF2BPs could influence the localization of mRNA
In the cytoplasm, IGF2BP1 was found to be present in RNP granules 200–700 nm in size and was distributed along microtubules; it moved at an average speed of 0.12 μm/s in an ATP-dependent manner.20 These granules were enriched in the perinuclear region but were also observed in neuronal processes and growth cones.126 Hence, these data supported the view that IGF2BP1 was associated with subcytoplasmic localization of the mRNA. Later, data from Jønson et al127 determined that the RNP granules were 100–300 nm in diameter and contained 40S ribosomal subunits, shuttling heterologous nuclear RNPs, poly(A) binding proteins, and mRNAs, as well as CBP80 and factors belonging to the exon junction complex without EIF4E, EIF4G, and 60S ribosomal subunits. Therefore, mRNAs integrated into IGF2BP1 mRNP particles could not be translated and transported to the appropriate destinations to initiate protein synthesis.
In human breast cancer, IGF2BP1 promoted the localization of mRNAs related to cell adhesion and motility, such as E-cadherin, β-actin, α-actinin, and Arp2/3, to stabilize cell–cell junctions and focal adhesions, which eventually suppress cancer cell invasion.128 However, this role of IGF2BP1 in stabilizing the cell junctions of breast cancer is contradictory to its role in cervical cancer, because Vikesaa et al129 showed that the deletion of IGF2BP1/3 resulted in the altered formation of invasive pseudopodia in the HeLa cell line. Complex tumorigenesis and development mechanisms led to the regulation of IGF2BP1 by different factors and binding to different mRNAs is one possible explanation.
IGF2BPs could regulate the translation of mRNA
In malignant cancers, another important function of IGF2BPs in mRNA processing is to regulate mRNA translation.130,131 Phosphorylation of IGF2BP2 by mTOR promotes its translational activity, thereby regulating the translation initiation of IGF2 leader 3 mRNA through EIF4E and activation of the 5′ cap-independent internal ribosome in human embryonic rhabdomyosarcoma.80 After identifying IGF2BP as m6A readers, IGF2BP1 was also shown to reduce BMI1 protein levels in oral squamous cell carcinoma (OSCC) as BMI1 m6A methylation levels decreased.12 Similarly, translational regulation of pancreatic and duodenal homeobox 1 (PDX1) in pancreatic β cells and V-Erb-B2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2) in radioiodine-refractory papillary thyroid cancer were also dependent on the recognition of m6A methylation by IGF2BP2.132,133 This evidence also provides strong evidence that IGF2BPs regulate mRNA translation through methylation of m6A.
However, just as the regulation of mRNA stability in malignant cancers by IGF2BP is twofold, so is the regulation of translation.134,135 As mentioned above, IGF2BPs participate in the cellular localization of β-actin. Hüttelmaier et al135 further studied its translation mechanism in neuroma cells and found that β-actin translation would occur only when IGF2BP1 transported it to the endpoint of mRNA transport and phosphorylated tyrosine residues on IGF2BP1 by protein kinase Src, which had blocked the translation of β-actin.
Other modulation mechanisms of IGF2BPs to RNA
In addition to the above functions, nuclear localization of IGF2BPs is also important. Rivera Vargas et al136 confirmed in six different cancer cell lines that the nucleocytoplasmic IGF2BP3–HNRNPM complex could regulate the expression of cyclins D1, D3, and G1 by shuttling to the nucleus and occupying the relevant binding sites before the export of mRNAs to the cytoplasm. In glioblastoma, IGF2BP2 could bind to oxidative phosphorylation-related mRNAs, such as NDUFS3 and COX7b, and deliver them to mitochondrial polysomes.137 These findings reveal the functional diversity of IGF2BPs in RNA processing.
Modulation of IGF2BPs for ncRNA
IGF2BPs not only affect mRNAs but also interact with ncRNAs, which in turn modulate the expression of malignant cancer-related RNAs (Table 3). As we know, ncRNAs can affect mRNAs involved in the biological process of tumorigenesis and help identify potential targets for cancer treatment, which contribute to the discovery of cancer drugs. Just as IGF2BPs are identified as new m6A readers, how they regulate lncRNAs and circRNAs is also being updated, which has been shown to be associated with m6A binding sites in ncRNAs. Understanding the mechanism by which IGF2BP regulates RNAs remains to be further clarified, in that they can prevent miRNA-dependent degradation of target RNAs; the only difference is that some RNA target RNAs have modified sites m6A that can be recognized and bound by IGF2BP.11 Therefore, we will summarize below the regulation network between IGF2BPs and ncRNAs and describe the specific mechanism.
Table 3.
The mechanism of IGF2BPs in regulating different ncRNAs in cancers.
| IGF2BPs | Cancer | ncRNAs | Mechanism of IGF2BPs in regulating ncRNAs | References |
|---|---|---|---|---|
| 1 | CRC | miR-183 | Prevent miR-183-dependent degradation of β-TrCP1 | 120 |
| 1 | Ovarian cancer | miR-155–5p, miR-22–3p, miR-140–3P | Prevent miRNA-dependent degradation of SIRT1 | 118 |
| 1 | HCC, ovarian cancer, lung cancer | miR-23a-3p, miR-125a-5p | Serve as an m6A reader and impair miRNA-dependent degradation of SRF | 11 |
| 1 | Breast cancer | lncRNA UCA1 | Recruit CCR4-NOT deadenylase complex to destabilize it | 148 |
| 1 | HCC | lncRNA HULC | Recruit CCR4-NOT deadenylase complex to destabilize it | 147 |
| 1 | HCC | circMAP3K4 | Promote translation | 149 |
| 2 | TC, NSCLC | lncRNA MALAT1 | Positive feedback may exist between IGF2BP2 and lncRNA MALAT1 | 66,145 |
| 2 | PC | lncRNA DANCR | Serve as an m6A reader to promote stabilization | 16 |
| 2 | CRC | lncRNA ZFAS1 | Serve as an m6A reader to promote stabilization | 144 |
| 2 | CRC | miR-195 | Prevent miRNA-dependent degradation of RAF-1 | 121 |
| 2 | Renal cancer | lncRNA DUXAP9 | Serve as an m6A reader to promote stabilization | 142 |
| 2 | Prostate cancer | lncRNA PCAT6 | Serve as an m6A reader to promote stabilization | 96 |
| 2 | Cervical cancer | circARHGAP12 | Serve as an m6A reader to promote stabilization | 107 |
| 3 | NPC | lncRNA TINCR | Slow its decay | 146 |
| 3 | Breast cancer | miR-3614 | Blockade of miR-3614 maturation | 139 |
| 3 | Cervical cancer | lncRNA KCNMB2-AS1 | Serve as an m6A reader to promote stabilization | 77 |
| 1/3 | HCC | LINC01138 | Promote it stabilization | 143 |
| 1/2/3 | Ovarian clear cell carcinoma/glioblastoma/some kinds of solid cancers and fibrosarcoma | let-7 | Prevent miRNA-dependent degradation of let-7 target mRNA | 116,117,119 |
CRC: colorectal cancer; HCC: hepatocellular carcinoma; NSCLC: non-small cell lung cancer; NPC: nasopharyngeal carcinoma; PC, pancreatic cancer; TC: thyroid cancer.
Interaction between IGF2BPs and miRNAs
One of the roles of miRNAs in cancers is to mediate mRNA silencing.138 As mentioned above, IGF2BPs alter the ability to decay miRNA-dependent mRNA by binding to the binding sites and attenuating the interaction between miRNAs and Ago2 or acting as cytoplasmic safe houses in the context of cancer.117, 118, 119, 120, 121,139 Based on the presence of m6A methylation sites in miRNA-regulated mRNAs in HCC, ovarian cancer, and lung cancer,11 we can continue to study whether most miRNA-targeted mRNAs have m6A sites, which can clarify the activity of IGF2BPs in cancers. Furthermore, combined activation of the IGF2BP family promoted oncogenic transformation through Dicer and was included in an oncogenic network that included miRNAs and complex post-transcriptional regulation.140 In this network, IGF2BPs regulated the interaction with miRNAs through a variety of complex mechanisms, thus affecting the expression of malignant cancer-related RNAs, and changing cell fate and behavior.
An important mechanism of IGF2BP1 affecting cancer was its complex regulation network with let-7 miRNA. Let-7 was a family of highly evolutionarily conserved miRNAs that suppressed cancer growth and is a key regulator of IGF2BP1 in post-transcriptional regulation. Although let-7 targets gene products, including LIN28A, LIN28B, and HMGA2, and displays oncogenic and self-renewal functions.141 The cancer suppressor role of the let-7 miRNA family was antagonized by the self-promoting oncogenic ‘triangle’ composed of HMGA2, IGF2BP1, and LIN28B.116 Similarly, IGF2BP2 protected let-7 miRNA family target genes from silencing to preserve glioblastoma stem cells.119 IGF2BP3 prevented the binding of Ago2/let-7 to LIN28B, thus increasing the expression of other let-7 target genes (such as HMGA2).117 The ultimate result of this complex regulation is to promote the occurrence and development of cancer.
Interaction between IGF2BPs and lncRNAs
LncRNAs are a subgroup of ncRNAs with a length of more than 200 nucleotides, which play an important role in various biological functions and disease processes. In the study of their cancer-promoting mechanisms, lncRNAs were found to be related to the methylation of m6A and could bind to IGF2BP to stabilize themselves.96,142,143 A representative example is the DANCR lncRNA regulated by IGF2BP2 in an m6A methylation-dependent manner, which promoted the stem cell-like properties of cancer, cell proliferation, and stabilization of PC pathogenesis.16 A recent study found that the crosstalk between IGF2BP2 and the ZFAS1 lncRNA promoted ATP hydrolysis and the Warburg effect and played an important role in mitochondrial energy metabolism in colon cancer.144 More importantly, lncRNAs can form positive feedback regulation with IGF2BPs to better promote cancer progression,66,77,145 meaning that the regulatory mechanism of IGF2BPs on lncRNA expression is complex. However, there is a study that has not investigated whether IGF2BP and lncRNA binding sites were modified by m6A methylation and only concluded that IGF2BP3 could slow lncRNA TINCR decay and promote its stability.146 However, IGF2BPs can also promote the destabilization of lncRNAs by recruiting the CCR4-NOT deadenylase complex, thus promoting the degradation of the lncRNA HULC in HCC and the lncRNA UCA1 in breast cancer.147,148 However, these data are also sufficient to demonstrate that one of the mechanisms of interaction between lncRNAs and IGF2BPs is through the m6A methylation, thus promoting cancer progression.
Interaction between IGF2BPs and circRNAs
Currently, there is little research on how IGF2BPs regulate circRNAs in cancer, and only a few articles have written that they function by recognizing and binding to the m6A site in circRNAs. For example, m6A methylation in circARHGAP12 is recognized by IGF2BP2, which then together promotes stabilization of the forkhead box M1 (FOXM1) mRNA in cervical cancer.107 The Hsa_circ_0003258–IGF2BP3–HDAC4 complex can enhance the stability of histone deacetylase 4 (HDAC4) mRNA, and both hsa_circ_0003258 and HDAC4 contain m6A modification sites.108 Interestingly, circMAP3K4 encodes a new peptide, and IGF2BP1 promotes its translation through m6A methylation.149 Apart from that, the elimination of circCD44 or IGF2BP2 influences the level of m6A-modified c-MYC mRNA, and the combination between circCD44 and IGF2BP2 may improve the stabilization of c-MYC in TNBC.73 Therefore, reasonable speculation is that IGF2BPs can bind to m6A-modified circRNAs, thus regulating the expression of downstream genes. However, that study also showed that IGF2BPs interact with circRNAs in more than one way. The KH3-4 di-domain of IGF2BP2 was also necessary for its interaction with circNSUN2 and HMGA2, which allowed IGF2BP2 to bind to the CAUCAU motif of circNSUN2, and then enhanced the stability of the HMGA2 mRNA. However, in this study, IGF2BP2 stabilized HMGA2 in a manner independent of m6A methylation.23
IGF2BPs could regulate the biological functions of cancer cells
CSCs are cells with self-renewal ability and cloning capacity, which are associated with cancer recurrence, metastasis, and resistance. Therefore, the mechanism of various factors that maintain the stem of CSCs needs to be studied in depth to identify targets that can be used for cancer therapy. IGF2BPs are reported to be key regulators of stem-like tumorigenic features in HCC, glioblastoma, and osteosarcoma.34,67,119,137 In leukemia, IGF2BP1 maintained the stemness of leukemia stem cells by regulating the key regulators of self-renewal, HOXB4, and MYB, and the metabolism-related factor ALDH1A1.150 Importantly, in a recent study, IGF2BP2 could recognize lncRNA DANCR and DROSHA mRNA through m6A methylation, thereby promoting the stemness of pancreatic or breast cancer stem cells.16,18 Disrupting the interaction between IGF2BP and MYC may suppress the stem properties of breast cancer cells.109 SRY box transcription factor 2 (SOX2) is also an important factor involved in maintaining the self-renewal capacity of CSCs.151 For IGF2BPs, IGF2BP2 stabilizes SOX2 in an m6A methylation-dependent manner to maintain the stem-like properties of CRC.152 Although IGF2BP1 also stabilized SOX2 in endometrial cancer,153 it remains to be explored whether IGF2BP1 promotes the maintenance of stemness. Unlike IGF2BP1/2, IGF2BP3 promoted cancer self-renewal and initiation by binding to SLUG and promoting its downstream target SOX2, rather than directly binding to SOX2 in TNBC.154 This is also evidence to support differences in the target RNAs of the IGF2BP family. In addition, in the hypoxic TME, IGF2BP also can contribute to maintaining the stem capacity of CSCs and adaptation to hypoxia.9,155
There is a period called the G1 phase between nuclear division (M phase) and DNA synthesis (S phase) of the cell cycle, allowing repair of DNA damage and replication errors.156 Mistakes in this period can lead to the formation of cancer cells. The regulation of the cancer cell cycle by IGF2BPs is positive and can promote the G1/S transition to promote cell proliferation.110,136,157, 158, 159 Subsequently, it was confirmed that IGF2BP3 could bind to m6A sites in the coding sequence (CDS) region of cyclin D1 (CCND1, a checkpoint of the G1/S phase of the cell cycle), increasing the proportion of cancer cells in phase S.160 In addition, we know that E2F is a positive regulator of the G1/S phase checkpoint, and IGF2BP1 promotes the shortening of the G1 phase by stabilizing its mRNA in a 3′UTR/miRNA/m6A methylation-dependent manner, thus promoting cancer cell proliferation.13 Cyclin-dependent kinases (CDK) are also a factor that promotes cell cycle entry into the S phase and can be a therapeutic target for cancer.161 Although the lncRNA DMDRMR could cooperate with IGF2BP3 to promote the transition G1/S by binding to the CDK4 m6A binding site.95
The generation of resistance to cancer drugs is a major challenge in cancer treatment. A review of the recent literature revealed that IGF2BPs can induce a variety of cancer cells to develop resistance to chemotherapeutic drugs, such as NPC,146 NSCLC,162 CRC,17 seminoma,163 leukemia,150 glioblastoma,164 and melanoma.165 Importantly, in radioiodine-refractory papillary thyroid carcinoma, IGF2BP2 promoted the translation efficiency of target RNAs through m6A methylation, thereby resulting in cancer cells resistant to tyrosine kinase inhibitors.133 Moreover, IGF2BPs are also factors that lead to cancer cells being resistant to radiotherapy.166,167
IGF2BPs regulate cancers by altering the TME
The TME is a complex environment in which cancer cells grow and includes multiple components, such as cancer-associated cells, the extracellular matrix, and some cytokines. However, due to metabolic disorders of cancer cells and other factors, an immunosuppressive TME is often formed and promotes cancer cells to evade immune surveillance, which is an important cause of the poor efficacy of current cancer therapy. Reviewing previous studies, it is not difficult to find that although some studies have confirmed that IGF2BPs can inhibit cancers, most studies support their cancer-promoting effects, such as inhibiting the antitumor immune response, promoting the function of immunosuppressive cells, adapting to hypoxia, and promoting cancer metabolism, angiogenesis, drug resistance, metastasis, and cell cycle transition. In the following, we summarize the specific roles of IGF2BPs in modulating TME, especially immunosuppressive TME.
IGF2BPs inhibit the anti-tumor immune response
With increasing research efforts, it has been found that IGF2BPs may be related to the immune response in the TME, resulting in dynamic changes in their impact on cancer cells. For example, a restricted epitope peptide derived from IGF2BP3 was found to induce CD8+ T cells to produce powerful and specific immune responses against cancer cells,168,169 suggesting that IGF2BP3 can serve as an antigen for T-cell-mediated immunotherapy. Although IGF2BP1-depleted TME could induce the appearance of CRC,170 which is inconsistent with the role of IGF2BP1 in the promotion of colon cancer found in other studies.89,171 This may be due to the different TME, but specific reasons still need to be further explored.
Given the few studies on IGF2BP and important cytokines in TME, and most are in inflammatory diseases,172,173 we next focus on the relationship between IGF2BP and tumor-associated cells. For antitumor immune cells, IGF2BP3 can stabilize PD-L1 mRNA expression through m6A methylation, thus inhibiting the killing effect of cytotoxic T cells in the TME,174 or inhibit NK cell-mediated cytotoxicity by promoting stress-induced ligand ULBP2 mRNA decay.124 CircIGF2BP3, a circRNA derived from a back splicing event between exons 4 and 13 of IGF2BP3, suppressed CD8+ T cell infiltration in NSCLC TME by promoting PD-L1 deubiquitination.175 In summary, IGF2BPs inhibit antitumor immune cells in the TME in various ways and ultimately promote tumor immune escape.
IGF2BPs promote the function of immunosuppressive cells
Immunosuppressive cells in the TME include tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), regulatory T cells (Treg), and carcinoma-associated fibroblasts (CAF). TAMs are formed by the infiltration of peripheral blood immune cells into cancer tissue and are also important factors that mediate cancer immune escape in the immunosuppressive TME. Among IGF2BPs, IGF2BP2 was involved in the immune response of peripheral blood immune cells in CRC patients,176 and the ternary complex composed of circITGB6/IGF2BP2/FGF9 can induce TAM polarization to the M2 phenotype (with tumor-promoting activity) in ovarian cancer.104 Because cell communication mediated by extracellular vesicles (EVs) is also a key part of TME, Pan et al88 found that after EVs secreted by glioma cells were delivered to TAMs, the transported circNEIL3 cargo could stabilize IGF2BP3 and promote the polarization of its immunosuppressive phenotype. In addition, EVs secreted by IGF2BP1 knockdown melanoma cells inhibit cancer cell metastasis,177 but it remains to be explored whether this process involves the regulation of immunosuppressive TME by IGF2BPs. In breast cancer, the co-culture of fibroblasts with cancer cells could promote the CAF phenotype, and under this condition, IGF2BP3 can promote the proliferation and survival of breast cancer cells by promoting CD44 expression in fibroblasts.178,179
In conclusion, although there are no studies on the relationship of IGF2BPs with other immunosuppressive cells, such as MDSCs and Tregs, continuing to dig deeper into this field may also help us understand tumorigenesis and deepen our understanding of the immunosuppressive TME.
IGF2BPs promote the adapting to hypoxia of cancer cells
Hypoxia caused by rapid cancer growth is a common feature of the TME, leading to increased expression of hypoxia-inducible factor (HIF), which promotes cancer immune escape. It has been confirmed that hypoxia could induce the invasion of metastatic melanoma cells by regulating IGF2BP1 expression through HIF-1α.36 Currently, only a few studies mention that IGF2BPs regulate the biological behavior of cancer cells in a hypoxic environment through m6A methylation. For example, in gastric cancer, IGF2BP3 recognizes the m6A site on HIF-1α mRNA and enhances its expression, thus promoting hypoxia-induced cell metastasis and angiogenesis.180 Further, hypoxia-induced downregulation of FTO promoted metastasis of CRC through an m6A-IGF2BP2-dependent mechanism.181 Whereas IGF2BP1 silencing in one study did not reverse hypoxia-induced chemoresistance in HCC,46 suggesting that IGF2BP1 was not the only gene involved in the regulation of chemoresistance. Importantly, binding of the lncRNA HIF1A-AS2 to IGF2BP2 promotes the adaptation of stem cells from glioblastoma multiforme to hypoxia.155 This adaptive change can prevent hypoxia-induced cancer cell necrosis and forms an immunosuppressive TME.
IGF2BPs could affect the cancer metabolism
Cancer cells can also change their metabolism to regulate the adaptation to hypoxia. The use of glycolysis as a source of energy even in the presence of oxygen, known as the Warburg effect, is a unique way of glucose metabolism in cancer cells. Lactic acid produced by cancer cells accumulates in the TME, resulting in an immunosuppressive effect. IGF2BPs can promote cancer glycolysis by interacting with target RNAs related to glucose metabolism.87,93,171,182, 183, 184 In studies on glucose metabolism in CRC, it was found that m6A methylation in target genes (HK2 and GLUT1) could promote the activation of glycolysis and cell proliferation, while IGF2BP2/3 could bind to them to play its m6A reader role.185 In addition, in gastric cancer (GC), HCC, and cervical cancer, there are similar findings that IGF2BP3 promotes the stability of target RNAs (HDGF, PDK4) through m6A methylation to regulate glycolysis and ATP production in cancer cells.14,186 In a recent study, Lu et al144 found that IGF2BP2 recognized and stabilized the lncRNA ZFAS1 through the m6A site, thus promoting ATP hydrolysis and the Warburg effect, providing a new mechanism for IGF2BP to promote cancer glycolysis. Although aerobic glycolysis is the main source for cancer cells to obtain energy, in recent years oxidative phosphorylation has also been found to be crucial in maintaining the stemness of some cancer stem cells (CSCs).187 IGF2BPs also play a role in this form of energy metabolism. For instance, inhibition of IGF2BP2-mediated oxidative phosphorylation was detrimental to the clonogenicity of glioblastoma CSC.137 However, because of the high energy demand of cancer cells, they often utilize lipid metabolism and amino acid metabolism to maintain their own growth needs. Current research on IGF2BP lipid metabolism has focused on its deletion which could make mice resistant to obesity and regulate glucose tolerance,188,189 but little is known about its mechanism of action in cancers. In NPC, IGF2BP3 could regulate acetyl-CoA-related metabolic processes by stabilizing the lncRNA TINCR, thus promoting lipid biosynthesis.146 It is believed that with the deepening of the research, the mechanism of m6A methylation mediated by IGF2BPs to promote cancer metabolism will be elucidated and warrants further research.
IGF2BPs could promote cancer neovascularization
Cancer neovascularization is very important for nutrient and oxygen delivery and is essential for cancer growth and metastasis. IGF2BPs can also use their m6A reader function to stabilize related mRNAs to promote cancer angiogenesis.14,42 Vascular endothelial growth factor (VEGF) is also an important regulator of angiogenesis. In recent years, through in-depth research on m6A methylation, we have learned that there are m6A sites in the VEGF mRNA, which could be recognized and bound by IGF2BP3, thus promoting cancer angiogenesis.160
In summary, the rapid growth of cancer cells leads to insufficient oxygen supply and the formation of a hypoxic environment, prompting cancer cells to alter their glucose metabolism and secrete a large amount of lactic acid into the TME, which inhibits the function of immune cells and enhances the activity of immunosuppressive cells. Moreover, the formation of cancer angiogenesis, cell cycle transition, and resistance to radiotherapy and chemotherapy, promote the cancer's adaptation to the adverse environment, which requires the joint efforts of all links. The role of IGF2BPs in the induction of immunosuppressive TME is very important. Due to the wide range of target RNAs of IGF2BPs, we can also find that they regulate many aspects of tumorigenesis and development, which may better explain the mechanism of IGF2BPs in the promotion of cancers. A better understanding of the mechanisms involved in IGF2BP regulation of immunosuppressive TME may contribute to avoiding cancer immune escape mechanisms.
The potential clinical application of IGF2BPs in cancers
Potential diagnosis values of IGF2BPs
The tumor-promoting effects of IGF2BPs have emerged from studies over the past few years. As we know, tissue biopsy is the gold standard for diagnosing cancer, and by studying the expression of IGF2BPs in tissues—using methods such as immunohistochemistry—it was found that they were rarely expressed in normal tissues. Therefore, whether IGF2BPs can be used as a cancer diagnostic marker is a question worthy of research (Table 4). For example, the detection of IGF2BP3 expression in samples obtained from a core needle biopsy or endoscopic biopsy could diagnose PC, CRC, etc.190, 191, 192, 193 In the immunohistochemical staining analysis of pleural effusion cell block, IGF2BP3 could be used to distinguish metastatic gastric adenocarcinoma cells and reactive mesothelial cells in effusions.194 However, IGF2BPs can not only distinguish between cancer and normal tissue but they can also differentiate between different types of cancers; IGF2BP1 can distinguish ATC from other follicular-derived thyroid cancers.33 With the development of medicine, the requirements for less invasive diagnostic methods are increasing, so the examination of markers in the peripheral blood of patients has attracted greater attention. Some researchers have studied whether the presence of anti-IGF2BP2 autoantibody in peripheral blood could be used as a basis for diagnosing CRC, but the results obtained were not ideal, a result which may be attributed to the very low sensitivity (23.4%), although its specificity was very high, reaching 97.1%. Therefore, more studies are needed to determine whether it could be used as a complementary marker to diagnose CRC.195
Table 4.
The diagnosis value of IGF2BPs in different cancers.
| Cancer type | IGF2BPs | Role of IGF2BPs in cancer diagnosis | Number of cases | References |
|---|---|---|---|---|
| Salivary gland tumors | 3 | Not a specific diagnostic marker for distinguishing salivary gland tumors | 36 | 196 |
| LSCC | 3 | Has good specificity and sensitivity for diagnosis of LSCC, but could not differentiate between carcinoma in situ and invasive carcinoma | 238 | 197 |
| ATC | 1 | Distinguishes ATC from another thyroid carcinoma of follicular origin | 365 | 33 |
| Follicular patterned thyroid tumors | 3 | Distinguishes malignant from benign follicular thyroid lesions | 219 | 251 |
| Papillary thyroid carcinoma | 3 | Inability to differentiate papillary carcinoma from benign lesions; sensitivity is 27% and specificity is 100% | 84 | 198 |
| Esophageal adenocarcinoma | 3 | Can diagnosis invasive esophageal adenocarcinoma and high-grade dysplasia | 217 + 76 | 252,253 |
| Extrapulmonary small cell carcinoma | 3 | Distinguishes small cell carcinoma from carcinoid tumor | 75 | 254 |
| Pancreatic carcinoma | 3 | A sensitive and specific marker for pancreatic ductal carcinoma and high-grade dysplastic lesions | 72 | 255 |
| PDAC | 3 | Distinguishes PDAC from chronic sclerosing pancreatitis and can be used in core needle biopsies; sensitivity is 88.4% and specificity is 94.6% | 240 | 190 |
| Malignant pancreatic cancers | 3 | Combined with IGF2BP3 immunostaining, the sensitivity, specificity, and accuracy of cytohistological analysis significantly increased to 87.9%, 100%, and 90.8% | 215 | 191 |
| Intraductal papillary mucinous neoplasm of the pancreas | 3 | Sensitivity for distinguishing cancerous from noncancerous lesions is 76.1% and specificity is 100% | 205 | 192 |
| Gastric adenocarcinoma | 3 | Distinguishes metastatic adenocarcinoma cells from reactive mesothelial cells in effusions; sensitivity is 78.4% and specificity is 92.5% | 156 | 194 |
| HCC | 3 | Seem to be of limited use as a single marker for the diagnosis; sensitivity is 52% and specificity is 97.1% | 452 | 199 |
| Cholangiocarcinoma | 3 | Distinguishes biliary cancer from the benign specimen (sensitivity is 76.4% and specificity is 80.9%); sensitivity is 89.7% and specificity is 91.7% when using IGF2BP3 and/or histology | 119 | 256 |
| Cholangiocarcinoma | 3 | Distinguishes biliary cancer from the benign specimen (sensitivity is 69.2% and accuracy is 80.0%); sensitivity is 80.8% and accuracy is 87.5% when combining IGF2BP3, EZH2, and p53 | 51 | 257 |
| Cholangiocarcinoma | 3 | Diagnosing the presence of invasion in bile duct biopsies | 37 | 258 |
| Extrahepatic bile duct carcinoma | 3 | Useful in the diagnosis of extrahepatic bile duct carcinoma; sensitivity is 79.4% and specificity is 91.7% | 80 | 259 |
| CRC | 2 | The specificity of anti-IGF2BP2 autoantibodies in serum to detect colon cancer is 97.1%, but the sensitivity is only 23.4% | 140 | 195 |
| CRC | 3 | Combination of histological features and IGF2BP3 increase the sensitivity (95.7%) and negative predictive values (61.1%) for detecting CRC in biopsy specimens | 1131 | 193 |
| Pelvic serous carcinoma | 3 | Could serve as a latent precancer biomarker | 316 | 260 |
| Endometrial carcinoma | 3 | IGF2BP3 together with L1CAM represent the optimal combination for discrimination between low- and high-grade endometrial carcinoma compared with IGF2BP3 or L1CAM alone | 378 | 261 |
| Teratoma | 3 | Might help diagnose metastatic mature teratoma and seminoma | 178 | 262 |
| Teratoma | 3 | Useful in the diagnosis of benign and malignant teratoma | 37 | 263 |
| Serous tubal carcinoma | 3 | Served as a complimentary biomarker to the diagnosis of serous tubal intraepithelial carcinoma | 170 | 264 |
| B-all | 1 | 95% sensitivity and 86% specificity for the diagnosis of ETV6-RUNX1 translocation-positive B-ALL | 143 | 265 |
| Angiosarcoma | 3 | Distinguish between malignant and benign vascular lesions | 71 | 266 |
| Hodgkin lymphoma | 3 | Served as a supplemental diagnostic marker | 81 | 267 |
| Hodgkin lymphoma | 3 | The sensitivity of IGF2BP3 to distinguish Hodgkin lymphoma is 84.3%; CD30/IGF2BP3 differentiates Hodgkin lymphoma with the same sensitivity as CD15/CD30 (traditional markers) | 51 | 268 |
| Leiomyosarcoma | 3 | Distinguishes leiomyoma from leiomyosarcoma | 216 | 269 |
| Chondrosarcoma | 3 | Differentiates problematic cases of enchondroma from well-differentiated chondrosarcomas | 78 | 270 |
ATC: anaplastic thyroid carcinomas; CRC: colorectal cancer; HCC: hepatocellular carcinoma; LSCC: laryngeal squamous cell carcinoma; PDAC: pancreatic ductal adenocarcinoma.
High specificity is a condition that can be used to define a cancer diagnostic marker, while the diagnostic utility of IGF2BPs in some cancers is still relatively low. The inability to differentiate salivary gland cancers,196 in situ carcinoma, and invasive laryngeal squamous cell carcinoma (LSCC),197 as well as benign lesions and malignant papillary thyroid cancer (PTC),198 or low diagnostic sensitivity in HCC,199 were all IGF2BP defects as diagnostic markers in different cancers. Therefore, greater efforts are needed to apply IGF2BP as a potential cancer diagnostic marker, and studies of IGF2BP1/2 as diagnostic markers are far less than those of IGF2BP3, indicating that more representative studies with larger samples are needed.
The prognosis value of IGF2BPs
How to accurately estimate the prognosis of various malignancies is always of interest to clinicians. Over the past few decades, an increasing number of studies on IGF2BPs have found that they are tumor-promoting factors, and their qualified potential as prognostic markers can be confirmed in various large-sample studies or bioinformatic analyses. IGF2BP3 is currently the most studied, and there is much literature on its prediction of a poor prognosis in different cancers. In contrast, although IGF2BP1/2 have been poorly studied, they are known to be associated with a poor prognosis in some common cancers (Table 5).
Table 5.
The prognosis value of IGF2BPs in cancers.
| System | Cancer type | IGF2BPs | Role of IGF2BPs in prognosis | References |
|---|---|---|---|---|
| Head and neck | HNSCC | 1 | Antibody responses to IGF2BP1 have a poor prognosis | 271 |
| HNSCC | 2 | Associated with T stage and poor prognosis | 272 | |
| OSCC, TSCC, NPC | 3 | Predicts poor outcome | 40,273,274 | |
| Endocrine system | PTC | 2 | A four-m6A-regulator signature including IGF2BP2 predicts poor prognosis | 210 |
| TC | 2 | A protective factor in the six-gene risk signature | 216 | |
| Poorly differentiate thyroid cancer | 3 | Associated with an increased risk of death, metastases, and DFS | 275 | |
| Respiratory system | NSCLC | 1 | Associated with male, cancer size, non-adenocarcinoma, smoking history, and poor prognosis | 200 |
| Lung cancer | 1 | A three-m6A-regulator signature including IGF2BP1 is related to OS, cancer status, and some clinical traits (gender, smoking history, etc.) | 208 | |
| LUAD | 3 | High expression of IGF2BPs predicts poorer OS and DFS | 276,277 | |
| Breast | Breast cancer | 1 | An independent prognostic factor; associated with shorter OS | 278 |
| Breast cancer | 2/3 | IGF2BP2/3, YTHDC2, and RBM15 could be used for the prognostic stratification of luminal A/B subtypes | 279 | |
| TNBC | 3 | Associated with a more aggressive phenotype and decreased OS | 280 | |
| Malignant phyllodes tumor of the breast | 3 | Associated with shorter periods of metastasis-free and DFS | 281 | |
| Metaplastic breast carcinoma | 3 | Related to poor OS | 282 | |
| Digestive system | ESCC | 3 | Associated with adverse clinical outcomes in patients treated with surgery alone | 283 |
| Esophageal adenocarcinoma | 3 | Associated with depth of cancer infiltration, lymph node metastases, and worse outcome | 202 | |
| Pancreatic cancer | 1/2 | Associated with poor prognosis | 44,45 | |
| Pancreatic ductal adenocarcinoma | 3 | Related to poor OS | 284 | |
| Intraductal papillary mucinous neoplasm | 3 | High expression of IGF2BP3 is related to shorter disease-specific survival | 192 | |
| Gastric adenocarcinoma | 3 | Predicts postoperative peritoneal dissemination and poor prognosis | 285 | |
| GC | 1 | High IGF2BP1 mRNA expression has poorer OS | 201 | |
| GC | 2 | Gene polymorphisms might be an independent predictor of chemotherapeutic response in patients with metastatic GC | 286 | |
| GC | 3 | Associated with poor disease-specific survival | 48 | |
| CRC | 1/2 | An independent poor prognostic marker | 17,152 | |
| HCC | 2 | Might be an independent risk factor | 32 | |
| HCC | 3 | Predicts early recurrence and poor prognosis | 203 | |
| Intrahepatic cholangiocarcinoma | 3 | Provides an independent prognostic value | 35 | |
| Gallbladder adenocarcinoma | 3 | Associated with high histological grade, advanced stage, lymphatic invasion, and worse OS | 287 | |
| Adenocarcinoma of the ampulla of Vater | 3 | Independently predicts shorter recurrence-free and OS | 288 | |
| Reproductive system | Endometrial cancer | 1 | High expression is associated with poor prognosis | 159 |
| Ovarian cancer | 1 | A three-m6A-regulator including IGF2BP1 signature predicts poor prognosis | 207 | |
| Ovarian clear cell carcinoma | 3 | Related to poor OS | 289 | |
| Uterine cancer | 1 | A pan-prognostic regulator | 290 | |
| Uterine corpus endometrial carcinoma | 3 | A three-m6A-regulator signature including IGF2BP3 predicts a poor prognosis | 209 | |
| Testicular germ cell tumor | 1 | A six-m6A-regulator signature including IGF2BP1 predicts a poor prognosis | 291 | |
| Prostate cancer | 3 | Has no independent prognostic value | 213,214 | |
| Prostate cancer | 3 | High IGF2BP3 serum levels are related to patients' poor prognosis | 292 | |
| Urinary system | ccRCC | 2 | The six-gene signature including IGF2BP2 is an independent risk factor for OS | 293 |
| ccRCC | 3 | An independent risk factor for localized CCRCC patients | 205 | |
| ccRCC | 3 | High co-expression of DMDRMR and IGF2BP3 is associated with poor outcomes | 95 | |
| Primary papillary renal cell carcinoma and ccRCC | 3 | An independent prognostic biomarker for identifying patients with distant metastasis risk | 294 | |
| Renal cell carcinoma | 3 | An independent prognostic biomarker related to metastasis and reduced 5-year OS | 204 | |
| Bladder cancer | 1/3 | Significantly associated with poor prognosis | 110,295 | |
| Aggressive urothelial carcinoma of the bladder | 3 | An independent prognostic biomarker; can identify patients who may benefit from early aggressive therapy | 296 | |
| Upper tract urothelial carcinoma | 3 | Independently associated with disease recurrence, cancer-specific mortality, and all-cause mortality | 297 | |
| Urachal carcinoma of the bladder | 3 | Has no prognostic significance | 212 | |
| Nervous system | Neuroblastoma | 1/3 | Predicts poorer prognosis | 206,298 |
| Neuroblastoma | 2 | A protective factor in the five-gene signature | 215 | |
| Glioma | 2 | Associated with poor OS and DFS | 62 | |
| Glioblastoma | 3 | A nine-gene signature is an independent risk factor for OS | 211 | |
| Hematopoietic system | AML, diffuse large b-cell lymphoma | 1/2/3 | Predicts poor OS | 299 |
| B-ALL | 3 | Portends a favorable survival high-risk B-ALL | 300 | |
| Skin | Melanoma | 3 | Predicts poor prognosis | 301 |
| Neuroendocrine tumor | Lung neuroendocrine tumor | 3 | Related to poor OS and DFS | 302 |
| Sarcoma | Osteosarcoma | 1 | Associated with high tumor grade, metastasis, recurrence, and poor response to chemotherapy | 303 |
| Osteosarcoma | 2 | An independent risk factor for OS and DFS | 304 | |
| Soft-tissue sarcoma | 1 | Patients with loss of IGF2BP1 have poorer DFS | 217 | |
| Uterine leiomyosarcoma | 3 | An independent risk factor | 305 | |
| Ewing sarcoma | 3 | Associated with poor OS | 91,249 |
B-ALL: B cell-acute lymphoblastic leukemia; ccRCC: clear cell renal carcinoma; CRC: colorectal cancer; ESCC: esophageal squamous cell carcinoma; GC: gastric cancer; HCC: hepatocellular carcinoma; HNSCC: head and neck squamous cell carcinoma; LUAD: lung adenocarcinoma; NPC: nasopharyngeal carcinoma; NSCLC: non-small cell lung cancer; OSCC, oral squamous cell carcinoma; PTC: papillary thyroid cancer; TC: thyroid cancer; TNBC: triple-negative breast cancer.
As shown in Table 5, several large-sample studies have analyzed the expression of IGF2BPs in different cancers and correlated levels with clinical information and revealed that IGF2BP expression may be associated with metastasis, depth of invasion, early recurrence, and shorter overall survival (OS), and disease-free survival (DFS), which are independent prognostic factors predicting a poor prognosis.95,200, 201, 202, 203, 204, 205, 206 For example, in a retrospective study, it was found that the 5-year metastasis-free survival rate of IGF2BP3-positive patients with renal cell carcinoma was much lower than that of IGF2BP3-negative patients, concluding that IGF2BP3 could help identify patients with high metastatic potential and who may benefit from early systemic therapy.204 However, in stage 4 neuroblastoma, researchers found that IGF2BP1 had prognostic significance independent of that of the MYC family member, MYCN.206 With the development of high-throughput sequencing and bioinformatics, various clinical information data sets can be analyzed to identify whether a gene is associated with cancer prognosis and to establish cancer risk prognosis models. In recent studies, IGF2BPs, as m6A readers, were selected from different models of different cancers, which could predict cancer prognostic information.207, 208, 209 IGF2BP2 was the only m6A gene that was correlated with the DFS of PTC and had a strong correlation with clinical phenotypes.210 In isocitrate dehydrogenase wild-type glioblastoma, Johnson et al211 established a nine-gene expression-based risk signature based on nine genes that included IGF2BP3, which predicted a poor prognosis.
However, evidence from some studies suggested that the prognosis of some cancers was not related to IGF2BP3 expression, such as urachal carcinoma of the bladder212 and prostate cancer.213,214 Even in several bioinformatics studies, IGF2BP2 was found to be a protective factor in neuroblastoma and TC,215,216 and deletion of IGF2BP1 led to poorer OS in soft tissue sarcomas.217 The reasons for this phenomenon may be due to the different types of cancers studied on the one hand and may be related to limited sample size or limitations of bioinformatics methods on the other. But these results also suggest that IGF2BP can also estimate the prognosis of certain cancers.
Therefore, IGF2BPs have great potential to predict cancer prognosis, involving different systems for a wide range of cancers.
The potential treatment values of IGF2BPs
The treatment of malignant cancers poses a significant challenge in the development of modern medicine. Given that cytotoxic T lymphocytes (CTL) respond to cancer cells expressing IGF2BP3.168,169,218 In small-cell lung cancer, Zhang et al219 constructed a prognostic signature based on m6A regulators, including IGF2BP3, the low score group had greater CD8+ T cell infiltration and better response to immunotherapy, so investigating immunotherapeutic approaches targeting IGF2BP3 in cancers is also an option. A recent phase I clinical trial demonstrated that a vaccine derived from IGF2BP3 and two other cancer peptides was effective against solid pediatric refractory cancers.220 As we know, antibody-based PD-1-PD-L1 inhibitors are an effective therapeutic approach for patients with various advanced cancers, but there are still many limitations and shortcomings. However, by further exploring the upstream regulatory mechanism of PD-L1, it was found that IGF2BP3 could stabilize its mRNA.174 This may help improve the effect of anti-PD-1 immunotherapy on cancers.
Because IGF2BPs promote the development of various cancers, the identification of specific inhibitors of IGF2BPs is also a viable approach to cancer treatment. In a recent study, a fluorescence polarization-based screening platform identified some specific inhibitors targeting IGF2BP2 belonging to the benzamidobenzoic acid class and the ureidothiophene class, and achieved reduced cancer xenograft growth in zebrafish embryos.221 This was the first description of a small-molecule inhibitor of IGF2BP2, facilitating the construction of RBP-specific inhibitors. Over the past few years, two inhibitors have been identified that specifically inhibit IGF2BP1. One is a newly identified small molecule inhibitor 7773, which binds directly to the hydrophobic surface of the KH3-4 domain of IGF2BP1 and acts by suppressing its binding to KRAS mRNA, thus specifically inhibiting its tumor-promoting activity.222 Another is a small-molecule inhibitor BTYNB, which functions as a selective allosteric inhibitor of IGF2BP1 to decrease IGF2BP1 protein levels.223 Mechanistically, BTYNB impairs the interaction between IGF2BP1 and c-MYC or E2F1 mRNA. Its inhibitory effect on IGF2BP1 was demonstrated simultaneously in experimental cancer models, where cancer growth and peritoneal spread were inhibited.13,223
In addition to these small-molecule inhibitors, many compounds play a role in inhibiting IGF2BPs. Confluentin, a compound isolated from Albatrellus flettii, inhibits the interaction between IGF2BP1 and KRAS mRNA.224 Furthermore, triptolide inhibits lnc-THOR-IGF2BP1 signaling, causing the inhibition of NPC cell growth.225 Berberine down-regulates IGF2BP3 expression to inhibit the proliferation of CRC cells,226 and IGF2BP3 expression is also inhibited by bromodomain and extraterminal domain inhibitor JQ1, which influences Ewing sarcoma anchorage-independent cell growth.91
Furthermore, oligonucleotides are also feasible as a new class of drugs, with several oligonucleotide therapies already on the market.227 The development of oligonucleotides targeting IGF2BP as oncology drugs is also a strategic option, as it can inhibit IGF2BP1 binding to mRNA.228,229
In conclusion, exploring the role of IGF2BPs in cancer immunotherapy and the development of drugs that specifically inhibit IGF2BPs, such as small molecule inhibitors, molecular compounds, and oligonucleotides, can help improve clinical therapeutic outcomes of different cancers, and therefore has great therapeutic potential.
Discussion
With the development of high-throughput sequencing and bioinformatics, an increasing number of studies have evaluated m6A methylation. We know that m6A methylation is inseparable from the occurrence and development of cancers and its mechanism is very complex. This review explored the role of m6A new readers IGF2BP1/2/3 in cancer immunosuppressive TME. Various functional in vitro and in vivo studies provide strong evidence for the regulation of cancer cell fate by IGF2BP, which act as oncogenes to promote cancer cell proliferation, survival, metastasis, and invasiveness in almost all cancers analyzed thus far. In contrast, a small number of studies report that IGF2BPs have an inhibitory effect on cancers.170,230,231 The different origins of cancer cells, differences in the TME, or the dissimilarity between nonmetastatic cells and metastatic cells are potential influencing factors. However, the detailed reasons for the contradictory functions of IGF2BP are still unclear and more research is needed to reveal the underlying causes. Additionally, we also reviewed the mechanisms by which IGF2BPs regulate cancers and find that IGF2BPs affect tumorigenesis by regulating the activity of different RNAs, including mRNAs, miRNAs, lncRNAs, and circRNAs. The consequence of this regulation is to inhibit the function of antitumor immune cells, influence cancer metabolism and the cancer cell cycle, and promote cancer angiogenesis and resistance to various treatments, thus inducing the formation of an immunosuppressive TME, which finally contributes to tumor-immune escape.
Currently, the research of various cancer markers has become an important direction for the development of oncology and other disciplines. More specific, sensitive, and minimally invasive markers discovered in these studies could help clinicians better diagnose and analyze the prognosis of malignant cancers earlier. At the same time, the discovery of different cancer suppressor genes and oncogenes, as well as an in-depth study of tumorigenesis, development, and malignant behavior, can help us better develop personalized cancer treatment strategies. Many studies indicate that further study of IGF2BP expression and its functions may meet the clinical requirements of new biomarkers for the diagnosis and prognosis of patients with malignant cancers. The search for inhibitors targeting the interaction between IGF2BP and its target RNAs may also be an effective strategy for cancer treatment. However, from the above discussion, it is not difficult to see that there are still several unanswered questions to be solved.
Although IGF2BPs can specifically recognize cancers with high specificity, their low sensitivity in some cancers is also of concern. Therefore, it may be more feasible to combine IGF2BPs with other highly sensitive markers. At the same time, exploring whether IGF2BPs are promising biomarkers for the detection of different cancers or whether they may have a better diagnostic and prognostic value for a specific cancer type is also a factor to evaluate prior to clinical application. With the increase in the application of immunotherapy in recent years and the growing research on the relationship between IGF2BPs and PD-L1, it may be worthwhile to continue exploring this combination. Although specific small molecule inhibitors and clinical application of cancer vaccines have provided additional choices for patients with malignant cancers, the development of IGF2BP inhibitors and therapeutic cancer vaccines has just begun, and more evidence is needed to prove their efficacy and safety.
In conclusion, the complex relationship between IGF2BPs and their target RNAs is involved in the development of cancer, especially in the formation of the immunosuppressive TME, which is likely to be accomplished by m6A methylation. Therefore, it may be possible to explore further interconnections between IGF2BPs and their target RNAs in the context of specific cancers, which may open new frontiers for the diagnosis, prognosis, and treatment of cancers.
Author contributions
Meiqi Duan drafted this review; Haiyang Liu provided editorial assistance and gave some valuable suggestions; Shasha Xu provided the manuscript revision and figure drawing; Xiaofeng Jiang and Shan Zhao provided the design, guidance and revision of the manuscript; Xiaofeng Jiang, Haiyang Liu and Zhi Yang obtained funding supports. All authors provided substantial and useful assistance to this manuscript; All authors approved the final manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This study was supported by grants from the Liaoning Nature Science Foundation of China (No. 2022JH2/101300042), The National Natural Science Foundation of China (No. 82173194, 81672877), the Key Research Project of Liaoning Provincial Department of Education of China (No. ZD2020004), and 2018 Youth Backbone Support Program of China Medical University (No. QGZ2018063).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Shan Zhao, Email: zhaoshan1109@163.com.
Xiaofeng Jiang, Email: 18900912171@163.com.
References
- 1.Mohammad H.P., Barbash O., Creasy C.L. Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer. Nat Med. 2019;25(3):403–418. doi: 10.1038/s41591-019-0376-8. [DOI] [PubMed] [Google Scholar]
- 2.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149(7):1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He L., Li H., Wu A., Peng Y., Shu G., Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18(1):176. doi: 10.1186/s12943-019-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H., Weng H., Sun W., et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müeller-Pillasch F., Lacher U., Wallrapp C., et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14(22):2729–2733. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 6.Mateu-Regué À., Christiansen J., Bagger F.O., Winther O., Hellriegel C., Nielsen F.C. Single mRNP analysis reveals that small cytoplasmic mRNP granules represent mRNA singletons. Cell Rep. 2019;29(3):736–748.e4. doi: 10.1016/j.celrep.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Weidensdorfer D., Stöhr N., Baude A., et al. Control of c-myc mRNA stability by IGF2BP1-associated cytoplasmic RNPs. RNA. 2009;15(1):104–115. doi: 10.1261/rna.1175909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell J.L., Wächter K., Mühleck B., et al. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs):post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70(15):2657–2675. doi: 10.1007/s00018-012-1186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu P., He F., Hou Y., et al. A novel hypoxic long noncoding RNA KB-1980E6.3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-Myc mRNA stability. Oncogene. 2021;40(9):1609–1627. doi: 10.1038/s41388-020-01638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F., Chen Z., Guan T., et al. N6-methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis. Cancer Res. 2021;81(11):2847–2860. doi: 10.1158/0008-5472.CAN-20-3779. [DOI] [PubMed] [Google Scholar]
- 11.Müller S., Glaß M., Singh A.K., et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47(1):375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L., Wu Y., Li Q., et al. METTL3 promotes tumorigenesis and metastasis through BMI1 m6A methylation in oral squamous cell carcinoma. Mol Ther. 2020;28(10):2177–2190. doi: 10.1016/j.ymthe.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller S., Bley N., Busch B., et al. The oncofetal RNA-binding protein IGF2BP1 is a druggable, post-transcriptional super-enhancer of E2F-driven gene expression in cancer. Nucleic Acids Res. 2020;48(15):8576–8590. doi: 10.1093/nar/gkaa653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Chen C., Ding Q., et al. METTL3-mediated m6A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69(7):1193–1205. doi: 10.1136/gutjnl-2019-319639. [DOI] [PubMed] [Google Scholar]
- 15.Cai X., Chen Y., Man D., et al. RBM15 promotes hepatocellular carcinoma progression by regulating N6-methyladenosine modification of YES1 mRNA in an IGF2BP1-dependent manner. Cell Death Dis. 2021;7(1):315. doi: 10.1038/s41420-021-00703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X., Peng W.X., Zhou H., et al. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 2020;27(6):1782–1794. doi: 10.1038/s41418-019-0461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H.M., Lin C.C., Chen W.S., et al. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) is a prognostic biomarker and associated with chemotherapy responsiveness in colorectal cancer. Int J Mol Sci. 2021;22(13):6940. doi: 10.3390/ijms22136940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng F., Xu J., Cui B., et al. Oncogenic AURKA-enhanced N6-methyladenosine modification increases DROSHA mRNA stability to transactivate STC1 in breast cancer stem-like cells. Cell Res. 2021;31(3):345–361. doi: 10.1038/s41422-020-00397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia M., Gut H., Chao J.A. Structural basis of IMP3 RRM12 recognition of RNA. RNA. 2018;24(12):1659–1666. doi: 10.1261/rna.065649.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen F.C., Nielsen J., Kristensen M.A., Koch G., Christiansen J. Cytoplasmic trafficking of IGF-II mRNA-binding protein by conserved KH domains. J Cell Sci. 2002;115(pt 10):2087–2097. doi: 10.1242/jcs.115.10.2087. [DOI] [PubMed] [Google Scholar]
- 21.Chao J.A., Patskovsky Y., Patel V., Levy M., Almo S.C., Singer R.H. ZBP1 recognition of beta-actin zipcode induces RNA looping. Genes Dev. 2010;24(2):148–158. doi: 10.1101/gad.1862910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dagil R., Ball N.J., Ogrodowicz R.W., et al. IMP1 KH1 and KH2 domains create a structural platform with unique RNA recognition and re-modelling properties. Nucleic Acids Res. 2019;47(8):4334–4348. doi: 10.1093/nar/gkz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R.X., Chen X., Xia L.P., et al. N6-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10(1):4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackedenski S., Wang C., Li W.M., Lee C.H. Characterizing the interaction between insulin-like growth factor 2 mRNA-binding protein 1 (IMP1) and KRAS expression. Biochem J. 2018;475(17):2749–2767. doi: 10.1042/BCJ20180575. [DOI] [PubMed] [Google Scholar]
- 25.Rensburg G.V., Mackedenski S., Lee C.H. Characterizing the coding region determinant-binding protein (CRD-BP)-microphthalmia-associated transcription factor (MITF) mRNA interaction. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes M., van Rensburg G., Li W.M., et al. Molecular insights into the coding region determinant-binding protein-RNA interaction through site-directed mutagenesis in the heterogeneous nuclear ribonucleoprotein-K-homology domains. J Biol Chem. 2015;290(1):625–639. doi: 10.1074/jbc.M114.614735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer N.A., Hansen Tv, Byskov A.G., et al. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction. 2005;130(2):203–212. doi: 10.1530/rep.1.00664. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Xu S., Xu G., et al. Dynamic expression of m6A regulators during multiple human tissue development and cancers. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.629030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastian F.B., Roux J., Niknejad A., et al. The Bgee suite: integrated curated expression atlas and comparative transcriptomics in animals. Nucleic Acids Res. 2021;49(D1):D831–D847. doi: 10.1093/nar/gkaa793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaß M., Misiak D., Bley N., et al. IGF2BP1, a conserved regulator of RNA turnover in cancer. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.632219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panebianco F., Kelly L.M., Liu P., et al. THADA fusion is a mechanism of IGF2BP3 activation and IGF1R signaling in thyroid cancer. Proc Natl Acad Sci U S A. 2017;114(9):2307–2312. doi: 10.1073/pnas.1614265114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pu J., Wang J., Qin Z., et al. IGF2BP2 promotes liver cancer growth through an m6A-FEN1-dependent mechanism. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.578816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haase J., Misiak D., Bauer M., et al. IGF2BP1 is the first positive marker for anaplastic thyroid carcinoma diagnosis. Mod Pathol. 2021;34(1):32–41. doi: 10.1038/s41379-020-0630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueki A., Shimizu T., Masuda K., et al. Up-regulation of Imp 3 confers in vivo tumorigenicity on murine osteosarcoma cells. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y., Yang M., Jiang Z., et al. IMP3 expression is associated with poor outcome and epigenetic deregulation in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45(6):1184–1191. doi: 10.1016/j.humpath.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Craig E.A., Weber J.D., Spiegelman V.S. Involvement of the mRNA binding protein CRD-BP in the regulation of metastatic melanoma cell proliferation and invasion by hypoxia. J Cell Sci. 2012;125(pt 24):5950–5954. doi: 10.1242/jcs.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin W., Watanabe H., Peng S., et al. Dynamic epigenetic regulation by menin during pancreatic islet tumor formation. Mol Cancer Res. 2015;13(4):689–698. doi: 10.1158/1541-7786.MCR-14-0457. [DOI] [PubMed] [Google Scholar]
- 38.Gu W., Wells A.L., Pan F., Singer R.H. Feedback regulation between zipcode binding protein 1 and beta-catenin mRNAs in breast cancer cells. Mol Cell Biol. 2008;28(16):4963–4974. doi: 10.1128/MCB.00266-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleynen I., Brants J.R., Peeters K., et al. HMGA2 regulates transcription of the Imp 2 gene via an intronic regulatory element in cooperation with nuclear factor-kappaB. Mol Cancer Res. 2007;5(4):363–372. doi: 10.1158/1541-7786.MCR-06-0331. [DOI] [PubMed] [Google Scholar]
- 40.Du M., Peng Y., Li Y., et al. MYC-activated RNA N6-methyladenosine reader IGF2BP3 promotes cell proliferation and metastasis in nasopharyngeal carcinoma. Cell Death Dis. 2022;8(1):53. doi: 10.1038/s41420-022-00844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samanta S., Sharma V.M., Khan A., Mercurio A.M. Regulation of IMP3 by EGFR signaling and repression by ERβ: implications for triple-negative breast cancer. Oncogene. 2012;31(44):4689–4697. doi: 10.1038/onc.2011.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y.S., Shi B.W., Guo J.H., et al. microRNA-320b suppresses HNF4G and IGF2BP2 expression to inhibit angiogenesis and tumor growth of lung cancer. Carcinogenesis. 2021;42(5):762–771. doi: 10.1093/carcin/bgab023. [DOI] [PubMed] [Google Scholar]
- 43.Huang X., Huang M., Kong L., Li Y. miR-372 suppresses tumour proliferation and invasion by targeting IGF2BP1 in renal cell carcinoma. Cell Prolif. 2015;48(5):593–599. doi: 10.1111/cpr.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan B.S., Cheng M., Zhang L. Insulin-like growth factor 2 mRNA-binding protein 1 promotes cell proliferation via activation of AKT and is directly targeted by microRNA-494 in pancreatic cancer. World J Gastroenterol. 2019;25(40):6063–6076. doi: 10.3748/wjg.v25.i40.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu X., Yu Y., Zong K., Lv P., Gu Y. Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic cancer promotes cancer proliferation by activating the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2019;38(1):497. doi: 10.1186/s13046-019-1470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebucci M., Sermeus A., Leonard E., et al. miRNA-196b inhibits cell proliferation and induces apoptosis in HepG2 cells by targeting IGF2BP1. Mol Cancer. 2015;14:79. doi: 10.1186/s12943-015-0349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H.Y., Ha Thi H.T., Hong S. IMP2 and IMP3 cooperate to promote the metastasis of triple-negative breast cancer through destabilization of progesterone receptor. Cancer Lett. 2018;415:30–39. doi: 10.1016/j.canlet.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y., Huang T., Siu H.L., et al. IGF2BP3 functions as a potential oncogene and is a crucial target of miR-34a in gastric carcinogenesis. Mol Cancer. 2017;16(1):77. doi: 10.1186/s12943-017-0647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H., Dong S., Du J., et al. Analysis of miRNA-mRNA crosstalk in radiation-induced mouse thymic lymphomas to identify miR-486 as a critical regulator by targeting IGF2BP3 mRNA. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.574001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fawzy I.O., Hamza M.T., Hosny K.A., Esmat G., El Tayebi H.M., Abdelaziz A.I. miR-1275: a single microRNA that targets the three IGF2-mRNA-binding proteins hindering tumor growth in hepatocellular carcinoma. FEBS Lett. 2015;589(17):2257–2265. doi: 10.1016/j.febslet.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 51.Kouhkan F., Mobarra N., Soufi-Zomorrod M., et al. MicroRNA-129-1 acts as tumour suppressor and induces cell cycle arrest of GBM cancer cells through targeting IGF2BP3 and MAPK1. J Med Genet. 2016;53(1):24–33. doi: 10.1136/jmedgenet-2015-103225. [DOI] [PubMed] [Google Scholar]
- 52.Huang R.S., Zheng Y.L., Li C., Ding C., Xu C., Zhao J. MicroRNA-485-5p suppresses growth and metastasis in non-small cell lung cancer cells by targeting IGF2BP2. Life Sci. 2018;199:104–111. doi: 10.1016/j.lfs.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Jiang T., Li M., Li Q., et al. microRNA-98-5p inhibits cell proliferation and induces cell apoptosis in hepatocellular carcinoma via targeting IGF2BP1. Oncol Res. 2017;25(7):1117–1127. doi: 10.3727/096504016X14821952695683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu F.Y., Zhou S.J., Deng Y.L., et al. miR-216b is involved in pathogenesis and progression of hepatocellular carcinoma through HBx-miR-216b-IGF2BP2 signaling pathway. Cell Death Dis. 2015;6(3) doi: 10.1038/cddis.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo Y., Sun R., Zhang J., Sun T., Liu X., Yang B. miR-506 inhibits the proliferation and invasion by targeting IGF2BP1 in glioblastoma. Am J Transl Res. 2015;7(10):2007–2014. [PMC free article] [PubMed] [Google Scholar]
- 56.Qin X., Sun L., Wang J. Restoration of microRNA-708 sensitizes ovarian cancer cells to cisplatin via IGF2BP1/Akt pathway. Cell Biol Int. 2017;41(10):1110–1118. doi: 10.1002/cbin.10819. [DOI] [PubMed] [Google Scholar]
- 57.Waly A.A., El-Ekiaby N., Assal R.A., et al. Methylation in MIRLET7A3 gene induces the expression of IGF-II and its mRNA binding proteins IGF2BP-2 and 3 in hepatocellular carcinoma. Front Physiol. 2018;9:1918. doi: 10.3389/fphys.2018.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang R.J., Li J.W., Bao B.H., et al. microRNA-873 (miRNA-873) inhibits glioblastoma tumorigenesis and metastasis by suppressing the expression of IGF2BP1. J Biol Chem. 2015;290(14):8938–8948. doi: 10.1074/jbc.M114.624700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou X., Zhang C.Z., Lu S.X., et al. miR-625 suppresses tumour migration and invasion by targeting IGF2BP1 in hepatocellular carcinoma. Oncogene. 2016;35(38):5078. doi: 10.1038/onc.2016.61. [DOI] [PubMed] [Google Scholar]
- 60.Xie L., Li S., Jin J., et al. Genetic variant in miR-21 binding sites is associated with colorectal cancer risk. J Cell Mol Med. 2019;23(3):2012–2019. doi: 10.1111/jcmm.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding L., Wang L., Guo F. microRNA-188 acts as a tumour suppressor in glioma by directly targeting the IGF2BP2 gene. Mol Med Rep. 2017;16(5):7124–7130. doi: 10.3892/mmr.2017.7433. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y., Liu X., Cheng L., et al. Tumor suppressor microRNA-138 suppresses low-grade glioma development and metastasis via regulating IGF2BP2. OncoTargets Ther. 2020;13:2247–2260. doi: 10.2147/OTT.S232795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kugel S., Sebastián C., Fitamant J., et al. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell. 2016;165(6):1401–1415. doi: 10.1016/j.cell.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyerinas B., Park S.M., Murmann A.E., et al. Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of multidrug resistance 1. Int J Cancer. 2012;130(8):1787–1797. doi: 10.1002/ijc.26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andres S.F., Williams K.N., Plesset J.B., et al. IMP1 3' UTR shortening enhances metastatic burden in colorectal cancer. Carcinogenesis. 2019;40(4):569–579. doi: 10.1093/carcin/bgy153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye M., Dong S., Hou H., Zhang T., Shen M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Ther Nucleic Acids. 2021;23:1–12. doi: 10.1016/j.omtn.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding W.B., Wang M.C., Yu J., et al. HBV/pregenomic RNA increases the stemness and promotes the development of HBV-related HCC through reciprocal regulation with insulin-like growth factor 2 mRNA-binding protein 3. Hepatology. 2021;74(3):1480–1495. doi: 10.1002/hep.31850. [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Jiang X., Zou A., et al. circIGHG-induced epithelial-to-mesenchymal transition promotes oral squamous cell carcinoma progression via miR-142-5p/IGF2BP3 signaling. Cancer Res. 2021;81(2):344–355. doi: 10.1158/0008-5472.CAN-20-0554. [DOI] [PubMed] [Google Scholar]
- 69.Qiu L., Zheng L., Gan C., Deng W., Sun Y., Wang T. circBICD2 targets miR-149-5p/IGF2BP1 axis to regulate oral squamous cell carcinoma progression. J Oral Pathol Med. 2021;50(7):668–680. doi: 10.1111/jop.13156. [DOI] [PubMed] [Google Scholar]
- 70.Wu K., Wang X., Yu H., Yu Z., Wang D., Xu X. LINC00460 facilitated tongue squamous cell carcinoma progression via the miR-320b/IGF2BP3 axis. Oral Dis. 2022;28(6):1496–1508. doi: 10.1111/odi.13828. [DOI] [PubMed] [Google Scholar]
- 71.Rong Z., Wang Z., Wang X., Qin C., Geng W. Molecular interplay between linc 01134 and YY1 dictates hepatocellular carcinoma progression. J Exp Clin Cancer Res. 2020;39(1):61. doi: 10.1186/s13046-020-01551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao J., Lin L., Luo D., et al. Long noncoding RNA TRPM2-AS acts as a microRNA sponge of miR-612 to promote gastric cancer progression and radioresistance. Oncogenesis. 2020;9(3):29. doi: 10.1038/s41389-020-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J., Gao X., Zhang Z., et al. CircCD44 plays oncogenic roles in triple-negative breast cancer by modulating the miR-502-5p/KRAS and IGF2BP2/Myc axes. Mol Cancer. 2021;20(1):138. doi: 10.1186/s12943-021-01444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin P., Huang Y., Zhu P., Zou Y., Shao T., Ouyang W. CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem Biophys Res Commun. 2018;503(3):1570–1574. doi: 10.1016/j.bbrc.2018.07.081. [DOI] [PubMed] [Google Scholar]
- 75.Liu P., Zhao P., Li B., Xu D., Wang K. LncRNA PCAT6 regulated by YY1 accelerates the progression of glioblastoma via miR-513/IGF2BP1. Neurochem Res. 2020;45(12):2894–2902. doi: 10.1007/s11064-020-03138-4. [DOI] [PubMed] [Google Scholar]
- 76.Fen H., Hongmin Z., Wei W., et al. RHPN1-AS1 drives the progression of hepatocellular carcinoma via regulating miR-596/IGF2BP2 axis. Curr Pharmaceut Des. 2020;25(43):4630–4640. doi: 10.2174/1381612825666191105104549. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y., Wang D., Wu D., Zhang D., Sun M. Long noncoding RNA KCNMB2-AS1 stabilized by N6-methyladenosine modification promotes cervical cancer growth through acting as a competing endogenous RNA. Cell Transplant. 2020;29 doi: 10.1177/0963689720964382. 963689720964382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu J., Han S. Downregulation of LncRNA DARS-AS1 inhibits the tumorigenesis of cervical cancer via inhibition of IGF2BP3. OncoTargets Ther. 2021;14:1331–1340. doi: 10.2147/OTT.S274623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hosono Y., Niknafs Y.S., Prensner J.R., et al. Oncogenic role of THOR, a conserved cancer/testis long non-coding RNA. Cell. 2017;171(7) doi: 10.1016/j.cell.2017.11.040. 1559-1572.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai N., Rapley J., Angel M., Yanik M.F., Blower M.D., Avruch J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 2011;25(11):1159–1172. doi: 10.1101/gad.2042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai N., Ji F., Wright J., Minichiello L., Sadreyev R., Avruch J. IGF2 mRNA binding protein-2 is a tumor promoter that drives cancer proliferation through its client mRNAs IGF2 and HMGA1. Elife. 2017;6 doi: 10.7554/eLife.27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai N., Christiansen J., Nielsen F.C., Avruch J. mTOR complex 2 phosphorylates IMP1 cotranslationally to promote IGF2 production and the proliferation of mouse embryonic fibroblasts. Genes Dev. 2013;27(3):301–312. doi: 10.1101/gad.209130.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jia C., Tang H., Yang Y., et al. Ubiquitination of IGF2BP3 by E3 ligase MKRN2 regulates the proliferation and migration of human neuroblastoma SHSY5Y cells. Biochem Biophys Res Commun. 2020;529(1):43–50. doi: 10.1016/j.bbrc.2020.05.112. [DOI] [PubMed] [Google Scholar]
- 84.Huang Y.Y., Zhang C.M., Dai Y.B., et al. USP11 facilitates colorectal cancer proliferation and metastasis by regulating IGF2BP3 stability. Am J Transl Res. 2021;13(2):480–496. [PMC free article] [PubMed] [Google Scholar]
- 85.Li H., Wang D., Yi B., et al. SUMOylation of IGF2BP2 promotes vasculogenic mimicry of glioma via regulating OIP5-AS1/miR-495-3p axis. Int J Biol Sci. 2021;17(11):2912–2930. doi: 10.7150/ijbs.58035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li B., Zhu L., Lu C., et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat Commun. 2021;12(1):295. doi: 10.1038/s41467-020-20527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y., Lu J.H., Wu Q.N., et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18(1):174. doi: 10.1186/s12943-019-1105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan Z., Zhao R., Li B., et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol Cancer. 2022;21(1):16. doi: 10.1186/s12943-021-01485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu S., Wang J.Z., Chen D., et al. An oncopeptide regulates m6A recognition by the m6A reader IGF2BP1 and tumorigenesis. Nat Commun. 2020;11(1):1685. doi: 10.1038/s41467-020-15403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng M., Xie X., Han G., et al. YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood. 2021;138(1):71–85. doi: 10.1182/blood.2020009676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mancarella C., Pasello M., Ventura S., et al. Insulin-like growth factor 2 mRNA-binding protein 3 is a novel post-transcriptional regulator of ewing sarcoma malignancy. Clin Cancer Res. 2018;24(15):3704–3716. doi: 10.1158/1078-0432.CCR-17-2602. [DOI] [PubMed] [Google Scholar]
- 92.Yan Y., Huang P., Mao K., et al. Anti-oncogene PTPN13 inactivation by hepatitis B virus X protein counteracts IGF2BP1 to promote hepatocellular carcinoma progression. Oncogene. 2021;40(1):28–45. doi: 10.1038/s41388-020-01498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang X., Guo S., Wang S., et al. EIF4A3-induced circARHGAP29 promotes aerobic glycolysis in docetaxel-resistant prostate cancer through IGF2BP2/c-Myc/LDHA signaling. Cancer Res. 2022;82(5):831–845. doi: 10.1158/0008-5472.CAN-21-2988. [DOI] [PubMed] [Google Scholar]
- 94.Shen H., Zhu H., Chen Y., et al. ZEB1-induced LINC01559 expedites cell proliferation, migration and EMT process in gastric cancer through recruiting IGF2BP2 to stabilize ZEB1 expression. Cell Death Dis. 2021;12(4):349. doi: 10.1038/s41419-021-03571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu Y., Niu S., Wang Y., et al. DMDRMR-mediated regulation of m6A-modified CDK4 by m6A reader IGF2BP3 drives ccRCC progression. Cancer Res. 2021;81(4):923–934. doi: 10.1158/0008-5472.CAN-20-1619. [DOI] [PubMed] [Google Scholar]
- 96.Lang C., Yin C., Lin K., et al. m6 A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11(6):e426. doi: 10.1002/ctm2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng Y., Gao L., Cui G., Cao Y. LncRNA NEAT1 facilitates pancreatic cancer growth and metastasis through stabilizing ELF3 mRNA. Am J Cancer Res. 2020;10(1):237–248. [PMC free article] [PubMed] [Google Scholar]
- 98.Lv X., Huang H., Feng H., Wei Z. Circ-MMP2 (circ-0039411) induced by FOXM1 promotes the proliferation and migration of lung adenocarcinoma cells in vitro and in vivo. Cell Death Dis. 2020;11(6):426. doi: 10.1038/s41419-020-2628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang F., Xue X., Zheng L., et al. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014;281(3):802–813. doi: 10.1111/febs.12625. [DOI] [PubMed] [Google Scholar]
- 100.Wu D., He X., Wang W., Hu X., Wang K., Wang M. Long noncoding RNA SNHG12 induces proliferation, migration, epithelial-mesenchymal transition, and stemness of esophageal squamous cell carcinoma cells via post-transcriptional regulation of BMI1 and CTNNB1. Mol Oncol. 2020;14(9):2332–2351. doi: 10.1002/1878-0261.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang Q., Guo H., Wang S., et al. A novel circular RNA, circXPO1, promotes lung adenocarcinoma progression by interacting with IGF2BP1. Cell Death Dis. 2020;11(12):1031. doi: 10.1038/s41419-020-03237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bao G., Huang J., Pan W., Li X., Zhou T. Long noncoding RNA CERS6-AS1 functions as a malignancy promoter in breast cancer by binding to IGF2BP3 to enhance the stability of CERS6 mRNA. Cancer Med. 2020;9(1):278–289. doi: 10.1002/cam4.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang W., Cheng X., Wang T., Song X., Zheng Y., Wang L. LINC00467 promotes cell proliferation and metastasis by binding with IGF2BP3 to enhance the mRNA stability of TRAF5 in hepatocellular carcinoma. J Gene Med. 2020;22(3) doi: 10.1002/jgm.3134. [DOI] [PubMed] [Google Scholar]
- 104.Li H., Luo F., Jiang X., et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J Immunother Cancer. 2022;10(3) doi: 10.1136/jitc-2021-004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hou P., Meng S., Li M., et al. LINC00460/DHX9/IGF2BP2 complex promotes colorectal cancer proliferation and metastasis by mediating HMGA1 mRNA stability depending on m6A modification. J Exp Clin Cancer Res. 2021;40(1):52. doi: 10.1186/s13046-021-01857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang G.W., Chen Q.Q., Ma C.C., Xie L.H., Gu J. linc 01305 promotes metastasis and proliferation of esophageal squamous cell carcinoma through interacting with IGF2BP2 and IGF2BP3 to stabilize HTR3A mRNA. Int J Biochem Cell Biol. 2021;136 doi: 10.1016/j.biocel.2021.106015. [DOI] [PubMed] [Google Scholar]
- 107.Ji F., Lu Y., Chen S., et al. IGF2BP2-modified circular RNA circARHGAP12 promotes cervical cancer progression by interacting m6A/FOXM1 manner. Cell Death Dis. 2021;7(1):215. doi: 10.1038/s41420-021-00595-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu Y.Z., Lv D.J., Wang C., et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol Cancer. 2022;21(1):12. doi: 10.1186/s12943-021-01480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma F., Liu X., Zhou S., et al. Long non-coding RNA FGF13-AS1 inhibits glycolysis and stemness properties of breast cancer cells through FGF13-AS1/IGF2BPs/Myc feedback loop. Cancer Lett. 2019;450:63–75. doi: 10.1016/j.canlet.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 110.Xie F., Huang C., Liu F., et al. CircPTPRA blocks the recognition of RNA N6-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol Cancer. 2021;20(1):1–17. doi: 10.1186/s12943-021-01359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu T., Ran L., Zhao H., et al. Circular RNA circ-TNPO3 suppresses metastasis of GC by acting as a protein decoy for IGF2BP3 to regulate the expression of MYC and SNAIL. Mol Ther Nucleic Acids. 2021;26:649–664. doi: 10.1016/j.omtn.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He J., Zuo Q., Hu B., et al. A novel, liver-specific long noncoding RNA LINC01093 suppresses HCC progression by interaction with IGF2BP1 to facilitate decay of GLI1 mRNA. Cancer Lett. 2019;450:98–109. doi: 10.1016/j.canlet.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 113.Wei L., Ling M., Yang S., Xie Y., Liu C., Yi W. Long noncoding RNA NBAT1 suppresses hepatocellular carcinoma progression via competitively associating with IGF2BP1 and decreasing c-Myc expression. Hum Cell. 2021;34(2):539–549. doi: 10.1007/s13577-020-00464-1. [DOI] [PubMed] [Google Scholar]
- 114.Zhai S., Xu Z., Xie J., et al. Epigenetic silencing of LncRNA LINC00261 promotes c-myc-mediated aerobic glycolysis by regulating miR-222-3p/HIPK2/ERK axis and sequestering IGF2BP1. Oncogene. 2021;40(2):277–291. doi: 10.1038/s41388-020-01525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biswas J., Patel V.L., Bhaskar V., Chao J.A., Singer R.H., Eliscovich C. The structural basis for RNA selectivity by the IMP family of RNA-binding proteins. Nat Commun. 2019;10:4440. doi: 10.1038/s41467-019-12193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Busch B., Bley N., Müller S., et al. The oncogenic triangle of HMGA2, LIN28B and IGF2BP1 antagonizes tumor-suppressive actions of the let-7 family. Nucleic Acids Res. 2016;44(8):3845–3864. doi: 10.1093/nar/gkw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jønson L., Christiansen J., Hansen T.V.O., Vikeså J., Yamamoto Y., Nielsen F.C. IMP3 RNP safe houses prevent miRNA-directed HMGA2 mRNA decay in cancer and development. Cell Rep. 2014;7(2):539–551. doi: 10.1016/j.celrep.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 118.Müller S., Bley N., Glaß M., et al. IGF2BP1 enhances an aggressive tumor cell phenotype by impairing miRNA-directed downregulation of oncogenic factors. Nucleic Acids Res. 2018;46(12):6285–6303. doi: 10.1093/nar/gky229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Degrauwe N., Schlumpf T.B., Janiszewska M., et al. The RNA binding protein IMP2 preserves glioblastoma stem cells by preventing let-7 target gene silencing. Cell Rep. 2016;15(8):1634–1647. doi: 10.1016/j.celrep.2016.04.086. [DOI] [PubMed] [Google Scholar]
- 120.Elcheva I., Goswami S., Noubissi F.K., Spiegelman V.S. CRD-BP protects the coding region of βTrCP1 mRNA from miR-183-mediated degradation. Mol Cell. 2009;35(2):240–246. doi: 10.1016/j.molcel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye S., Song W., Xu X., Zhao X., Yang L. IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF-1 degradation by miR-195. FEBS Lett. 2016;590(11):1641–1650. doi: 10.1002/1873-3468.12205. [DOI] [PubMed] [Google Scholar]
- 122.Chen Y., Zhao Y., Chen J., et al. ALKBH5 suppresses malignancy of hepatocellular carcinoma via m6A-guided epigenetic inhibition of LYPD1. Mol Cancer. 2020;19(1):123. doi: 10.1186/s12943-020-01239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ennajdaoui H., Howard J.M., Sterne-Weiler T., et al. IGF2BP3 modulates the interaction of invasion-associated transcripts with RISC. Cell Rep. 2016;15(9):1876–1883. doi: 10.1016/j.celrep.2016.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmiedel D., Tai J., Yamin R., Berhani O., Bauman Y., Mandelboim O. The RNA binding protein IMP3 facilitates tumor immune escape by downregulating the stress-induced ligands ULPB2 and MICB. Elife. 2016;5 doi: 10.7554/eLife.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mizutani R., Imamachi N., Suzuki Y., et al. Oncofetal protein IGF2BP3 facilitates the activity of proto-oncogene protein eIF4E through the destabilization of EIF4E-BP2 mRNA. Oncogene. 2016;35(27):3495–3502. doi: 10.1038/onc.2015.410. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H.L., Eom T., Oleynikov Y., et al. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31(2):261–275. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]
- 127.Jønson L., Vikesaa J., Krogh A., et al. Molecular composition of IMP1 ribonucleoprotein granules. Mol Cell Proteomics. 2007;6(5):798–811. doi: 10.1074/mcp.M600346-MCP200. [DOI] [PubMed] [Google Scholar]
- 128.Gu W., Katz Z., Wu B., et al. Regulation of local expression of cell adhesion and motility-related mRNAs in breast cancer cells by IMP1/ZBP1. J Cell Sci. 2012;125(Pt 1):81–91. doi: 10.1242/jcs.086132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vikesaa J., Hansen T.V.O., Jønson L., et al. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J. 2006;25(7):1456–1468. doi: 10.1038/sj.emboj.7601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Faye M.D., Beug S.T., Graber T.E., et al. IGF2BP1 controls cell death and drug resistance in rhabdomyosarcomas by regulating translation of cIAP1. Oncogene. 2015;34(12):1532–1541. doi: 10.1038/onc.2014.90. [DOI] [PubMed] [Google Scholar]
- 131.Weinlich S., Hüttelmaier S., Schierhorn A., Behrens S.E., Ostareck-Lederer A., Ostareck D.H. IGF2BP1 enhances HCV IRES-mediated translation initiation via the 3'UTR. RNA. 2009;15(8):1528–1542. doi: 10.1261/rna.1578409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Regué L., Zhao L., Ji F., Wang H., Avruch J., Dai N. RNA m6A reader IMP2/IGF2BP2 promotes pancreatic β-cell proliferation and insulin secretion by enhancing PDX1 expression. Mol Metabol. 2021;48 doi: 10.1016/j.molmet.2021.101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sa R., Liang R., Qiu X., He Z., Liu Z., Chen L. IGF2BP2-dependent activation of ERBB2 signaling contributes to acquired resistance to tyrosine kinase inhibitor in differentiation therapy of radioiodine-refractory papillary thyroid cancer. Cancer Lett. 2022;527:10–23. doi: 10.1016/j.canlet.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 134.Stöhr N., Köhn M., Lederer M., et al. IGF2BP1 promotes cell migration by regulating MK5 and PTEN signaling. Genes Dev. 2012;26(2):176–189. doi: 10.1101/gad.177642.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hüttelmaier S., Zenklusen D., Lederer M., et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438(7067):512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 136.Rivera Vargas T., Boudoukha S., Simon A., et al. Post-transcriptional regulation of cyclins D1, D3 and G1 and proliferation of human cancer cells depend on IMP-3 nuclear localization. Oncogene. 2014;33(22):2866–2875. doi: 10.1038/onc.2013.252. [DOI] [PubMed] [Google Scholar]
- 137.Janiszewska M., Suvà M.L., Riggi N., et al. Imp 2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26(17):1926–1944. doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jonas S., Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16(7):421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 139.Wang Z., Tong D., Han C., et al. Blockade of miR-3614 maturation by IGF2BP3 increases TRIM25 expression and promotes breast cancer cell proliferation. EBioMedicine. 2019;41:357–369. doi: 10.1016/j.ebiom.2018.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.JnBaptiste C.K., Gurtan A.M., Thai K.K., et al. Dicer loss and recovery induce an oncogenic switch driven by transcriptional activation of the oncofetal Imp 1-3 family. Genes Dev. 2017;31(7):674–687. doi: 10.1101/gad.296301.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chirshev E., Oberg K.C., Ioffe Y.J., Unternaehrer J.J. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin Transl Med. 2019;8(1):24. doi: 10.1186/s40169-019-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tan L., Tang Y., Li H., et al. N6-methyladenosine modification of LncRNA DUXAP9 promotes renal cancer cells proliferation and motility by activating the PI3K/AKT signaling pathway. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.641833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Z., Zhang J., Liu X., et al. The LINC01138 drives malignancies via activating arginine methyltransferase 5 in hepatocellular carcinoma. Nat Commun. 2018;9(1):1572. doi: 10.1038/s41467-018-04006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu S., Han L., Hu X., et al. N6-methyladenosine reader IMP2 stabilizes the ZFAS1/OLA1 axis and activates the Warburg effect: implication in colorectal cancer. J Hematol Oncol. 2021;14(1):188. doi: 10.1186/s13045-021-01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Han L., Lei G., Chen Z., Zhang Y., Huang C., Chen W. IGF2BP2 regulates MALAT1 by serving as an N6-methyladenosine reader to promote NSCLC proliferation. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.780089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng Z.Q., Li Z.X., Guan J.L., et al. Long noncoding RNA TINCR-mediated regulation of acetyl-CoA metabolism promotes nasopharyngeal carcinoma progression and chemoresistance. Cancer Res. 2020;80(23):5174–5188. doi: 10.1158/0008-5472.CAN-19-3626. [DOI] [PubMed] [Google Scholar]
- 147.Hämmerle M., Gutschner T., Uckelmann H., et al. Posttranscriptional destabilization of the liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding protein 1 (IGF2BP1) Hepatology. 2013;58(5):1703–1712. doi: 10.1002/hep.26537. [DOI] [PubMed] [Google Scholar]
- 148.Zhou Y., Meng X., Chen S., et al. IMP1 regulates UCA1-mediated cell invasion through facilitating UCA1 decay and decreasing the sponge effect of UCA1 for miR-122-5p. Breast Cancer Res. 2018;20(1):32. doi: 10.1186/s13058-018-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Duan J.L., Chen W., Xie J.J., et al. A novel peptide encoded by N6-methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol Cancer. 2022;21(1):93. doi: 10.1186/s12943-022-01537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Elcheva I.A., Wood T., Chiarolanzio K., et al. RNA-binding protein IGF2BP1 maintains leukemia stem cell properties by regulating HOXB4, MYB, and ALDH1A1. Leukemia. 2020;34(5):1354–1363. doi: 10.1038/s41375-019-0656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Novak D., Hüser L., Elton J.J., Umansky V., Altevogt P., Utikal J. SOX2 in development and cancer biology. Semin Cancer Biol. 2020;67(pt 1):74–82. doi: 10.1016/j.semcancer.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 152.Li T., Hu P.S., Zuo Z., et al. METTL3 facilitates tumor progression via an m6A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(1):112. doi: 10.1186/s12943-019-1038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xue T., Liu X., Zhang M., et al. PADI2-catalyzed MEK1 citrullination activates ERK1/2 and promotes IGF2BP1-mediated SOX2 mRNA stability in endometrial cancer. Adv Sci. 2021;8(6) doi: 10.1002/advs.202002831. 2002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Samanta S., Sun H., Goel H.L., et al. IMP3 promotes stem-like properties in triple-negative breast cancer by regulating SLUG. Oncogene. 2016;35(9):1111–1121. doi: 10.1038/onc.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mineo M., Ricklefs F., Rooj A.K., et al. The long non-coding RNA HIF1A-AS2 facilitates the maintenance of mesenchymal glioblastoma stem-like cells in hypoxic niches. Cell Rep. 2016;15(11):2500–2509. doi: 10.1016/j.celrep.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432(7015):298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 157.Zhang J., Luo W., Chi X., et al. IGF2BP1 silencing inhibits proliferation and induces apoptosis of high glucose-induced non-small cell lung cancer cells by regulating Netrin-1. Arch Biochem Biophys. 2020;693 doi: 10.1016/j.abb.2020.108581. [DOI] [PubMed] [Google Scholar]
- 158.Xu Y., Zheng Y., Liu H., Li T. Modulation of IGF2BP1 by long non-coding RNA HCG11 suppresses apoptosis of hepatocellular carcinoma cells via MAPK signaling transduction. Int J Oncol. 2017;51(3):791–800. doi: 10.3892/ijo.2017.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang L., Wan Y., Zhang Z., et al. IGF2BP1 overexpression stabilizes PEG10 mRNA in an m6A-dependent manner and promotes endometrial cancer progression. Theranostics. 2021;11(3):1100–1114. doi: 10.7150/thno.49345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang Z., Wang T., Wu D., Min Z., Tan J., Yu B. RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer. J Exp Clin Cancer Res. 2020;39(1):203. doi: 10.1186/s13046-020-01714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sherr C.J., Beach D., Shapiro G.I. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6(4):353–367. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Han X., Chen L., Hu Z., et al. Identification of proteins related with pemetrexed resistance by iTRAQ and PRM-based comparative proteomic analysis and exploration of IGF2BP2 and FOLR1 functions in non-small cell lung cancer cells. J Proteonomics. 2021;237 doi: 10.1016/j.jprot.2021.104122. [DOI] [PubMed] [Google Scholar]
- 163.Wei J., Yin Y., Zhou J., et al. METTL3 potentiates resistance to cisplatin through m6 A modification of TFAP2C in seminoma. J Cell Mol Med. 2020;24(19):11366–11380. doi: 10.1111/jcmm.15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X., Li X., Zhou Y., Huang X., Jiang X. Long non-coding RNA OIP5-AS1 inhibition upregulates microRNA-129-5p to repress resistance to temozolomide in glioblastoma cells via downregulating IGF2BP2. Cell Biol Toxicol. 2022;38(6):963–977. doi: 10.1007/s10565-021-09614-z. [DOI] [PubMed] [Google Scholar]
- 165.Kim T., Havighurst T., Kim K., Albertini M., Xu Y.G., Spiegelman V.S. Targeting insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in metastatic melanoma to increase efficacy of BRAFV600E inhibitors. Mol Carcinog. 2018;57(5):678–683. doi: 10.1002/mc.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yoshino K., Motoyama S., Koyota S., et al. Identification of insulin-like growth factor 2 mRNA-binding protein 3 as a radioresistance factor in squamous esophageal cancer cells. Dis Esophagus. 2014;27(5):479–484. doi: 10.1111/j.1442-2050.2012.01415.x. [DOI] [PubMed] [Google Scholar]
- 167.Hao C.C., Xu C.Y., Zhao X.Y., et al. Up-regulation of VANGL1 by IGF2BPs and miR-29b-3p attenuates the detrimental effect of irradiation on lung adenocarcinoma. J Exp Clin Cancer Res. 2020;39(1):256. doi: 10.1186/s13046-020-01772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tomita Y., Harao M., Senju S., et al. Peptides derived from human insulin-like growth factor-II mRNA binding protein 3 can induce human leukocyte antigen-A2-restricted cytotoxic T lymphocytes reactive to cancer cells. Cancer Sci. 2011;102(1):71–78. doi: 10.1111/j.1349-7006.2010.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Suda T., Tsunoda T., Daigo Y., Nakamura Y., Tahara H. Identification of human leukocyte antigen-A24-restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci. 2007;98(11):1803–1808. doi: 10.1111/j.1349-7006.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hamilton K.E., Chatterji P., Lundsmith E.T., et al. Loss of stromal IMP1 promotes a tumorigenic microenvironment in the colon. Mol Cancer Res. 2015;13(11):1478–1486. doi: 10.1158/1541-7786.MCR-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang X.L., Li K.J., Feng J.X., Liu G.J., Feng Y.L. Blocking the IGF2BP1-promoted glucose metabolism of colon cancer cells via direct de-stabilizing mRNA of the LDHA enhances anticancer effects. Mol Ther Nucleic Acids. 2021;23:835–846. doi: 10.1016/j.omtn.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang X., Ji Y., Feng P., et al. The m6A reader IGF2BP2 regulates macrophage phenotypic activation and inflammatory diseases by stabilizing TSC1 and PPARγ. Adv Sci. 2021;8(13) doi: 10.1002/advs.202100209. 2100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bechara R., Amatya N., Bailey R.D., et al. The m6A reader IMP2 directs autoimmune inflammation through an IL-17- and TNFα-dependent C/EBP transcription factor axis. Sci Immunol. 2021;6(61) doi: 10.1126/sciimmunol.abd1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wan W., Ao X., Chen Q., et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N6-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer. 2022;21(1):60. doi: 10.1186/s12943-021-01447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Z., Wang T., She Y., et al. N6-methyladenosine-modified circIGF2BP3 inhibits CD8+ T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol Cancer. 2021;20(1):105. doi: 10.1186/s12943-021-01398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xie J., Huang Z., Jiang P., et al. Elevated N6-methyladenosine RNA levels in peripheral blood immune cells: a novel predictive biomarker and therapeutic target for colorectal cancer. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.760747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ghoshal A., Rodrigues L.C., Gowda C.P., et al. Extracellular vesicle-dependent effect of RNA-binding protein IGF2BP1 on melanoma metastasis. Oncogene. 2019;38(21):4182–4196. doi: 10.1038/s41388-019-0797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Strell C., Paulsson J., Jin S.B., et al. Impact of epithelial-stromal interactions on peritumoral fibroblasts in ductal carcinoma in situ. J Natl Cancer Inst. 2019;111(9):983–995. doi: 10.1093/jnci/djy234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Y., Yu C., Wu Y., et al. CD44+ fibroblasts increases breast cancer cell survival and drug resistance via IGF2BP3-CD44-IGF2 signalling. J Cell Mol Med. 2017;21(9):1979–1988. doi: 10.1111/jcmm.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jiang L., Li Y., He Y., Wei D., Yan L., Wen H. Knockdown of m6A reader IGF2BP3 inhibited hypoxia-induced cell migration and angiogenesis by regulating hypoxia inducible factor-1α in stomach cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.711207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ruan D.Y., Li T., Wang Y.N., et al. FTO downregulation mediated by hypoxia facilitates colorectal cancer metastasis. Oncogene. 2021;40(33):5168–5181. doi: 10.1038/s41388-021-01916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang S., Wu Z., Cheng Y., Wei W., Hao L. Insulin-like growth factor 2 mRNA binding protein 2 promotes aerobic glycolysis and cell proliferation in pancreatic ductal adenocarcinoma via stabilizing GLUT1 mRNA. Acta Biochim Biophys Sin. 2019;51(7):743–752. doi: 10.1093/abbs/gmz048. [DOI] [PubMed] [Google Scholar]
- 183.Huang J., Sun W., Wang Z., et al. FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J Exp Clin Cancer Res. 2022;41(1):42. doi: 10.1186/s13046-022-02254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.An M.X., Li S., Yao H.B., et al. BAG3 directly stabilizes Hexokinase 2 mRNA and promotes aerobic glycolysis in pancreatic cancer cells. J Cell Biol. 2017;216(12):4091–4105. doi: 10.1083/jcb.201701064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shen C., Xuan B., Yan T., et al. m6A-dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19(1):72. doi: 10.1186/s12943-020-01190-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Z., Peng Y., Li J., et al. N6-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat Commun. 2020;11(1):2578. doi: 10.1038/s41467-020-16306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sica V., Bravo-San Pedro J.M., Stoll G., Kroemer G. Oxidative phosphorylation as a potential therapeutic target for cancer therapy. Int J Cancer. 2020;146(1):10–17. doi: 10.1002/ijc.32616. [DOI] [PubMed] [Google Scholar]
- 188.Dai N., Zhao L., Wrighting D., et al. IGF2BP2/IMP2-deficient mice resist obesity through enhanced translation of Ucp1 mRNA and other mRNAs encoding mitochondrial proteins. Cell Metabol. 2015;21(4):609–621. doi: 10.1016/j.cmet.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tybl E., Shi F.D., Kessler S.M., et al. Overexpression of the IGF2-mRNA binding protein p62 in transgenic mice induces a steatotic phenotype. J Hepatol. 2011;54(5):994–1001. doi: 10.1016/j.jhep.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wachter D.L., Schlabrakowski A., Hoegel J., Kristiansen G., Hartmann A., Riener M.O. Diagnostic value of immunohistochemical IMP3 expression in core needle biopsies of pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2011;35(6):873–877. doi: 10.1097/PAS.0b013e3182189223. [DOI] [PubMed] [Google Scholar]
- 191.Senoo J., Mikata R., Kishimoto T., et al. Immunohistochemical analysis of IMP3 and p53 expression in endoscopic ultrasound-guided fine needle aspiration and resected specimens of pancreatic diseases. Pancreatology. 2018;18(2):176–183. doi: 10.1016/j.pan.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 192.Morimatsu K., Aishima S., Yamamoto H., et al. Insulin-like growth factor II messenger RNA-binding protein-3 is a valuable diagnostic and prognostic marker of intraductal papillary mucinous neoplasm. Hum Pathol. 2013;44(9):1714–1721. doi: 10.1016/j.humpath.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 193.Wei Q., Zhou H., Zhong L., et al. IMP3 expression in biopsy specimens as a diagnostic biomarker for colorectal cancer. Hum Pathol. 2017;64:137–144. doi: 10.1016/j.humpath.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 194.Kim H.J., Kim G.E., Lee J.S., Lee J.H., Nam J.H., Choi C. Insulin-like growth factor-II mRNA-binding protein 3 expression in effusion cytology: a marker for metastatic adenocarcinoma cells and a potential prognostic indicator in gastric adenocarcinoma. Acta Cytol. 2014;58(2):167–173. doi: 10.1159/000357199. [DOI] [PubMed] [Google Scholar]
- 195.Liu W., Li Z., Xu W., Wang Q., Yang S. Humoral autoimmune response to IGF2 mRNA-binding protein (IMP2/p62) and its tissue-specific expression in colon cancer. Scand J Immunol. 2013;77(4):255–260. doi: 10.1111/sji.12032. [DOI] [PubMed] [Google Scholar]
- 196.Ismerim A.B., Ferreira S.V., Lessa A.M., et al. Insulin-like growth factor II messenger RNA-binding protein 3 in salivary gland tumors. Appl Immunohistochem Mol Morphol. 2016;24(6):422–426. doi: 10.1097/PAI.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 197.Chen K., Cornejo K.M., Ye W., Wu Q., Liang J., Jiang Z. Oncofetal protein IMP3: a new diagnostic biomarker for laryngeal carcinoma. Hum Pathol. 2013;44(10):2126–2131. doi: 10.1016/j.humpath.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 198.Yorukoglu A., Yalcin N., Avci A., et al. Significance of IMP3, nucleophosmin, and Ki-67 expression in papillary thyroid carcinoma. Int J Surg Pathol. 2015;23(1):5–12. doi: 10.1177/1066896914554832. [DOI] [PubMed] [Google Scholar]
- 199.Wachter D.L., Kristiansen G., Soll C., et al. Insulin-like growth factor II mRNA-binding protein 3 (IMP3) expression in hepatocellular carcinoma. A clinicopathological analysis with emphasis on diagnostic value. Histopathology. 2012;60(2):278–286. doi: 10.1111/j.1365-2559.2011.04091.x. [DOI] [PubMed] [Google Scholar]
- 200.Kato T., Hayama S., Yamabuki T., et al. Increased expression of insulin-like growth factor-II messenger RNA-binding protein 1 is associated with tumor progression in patients with lung cancer. Clin Cancer Res. 2007;13(2 Pt 1):434–442. doi: 10.1158/1078-0432.CCR-06-1297. [DOI] [PubMed] [Google Scholar]
- 201.Wang X., Guan D., Wang D., et al. Genetic variants in m6A regulators are associated with gastric cancer risk. Arch Toxicol. 2021;95(3):1081–1088. doi: 10.1007/s00204-020-02958-1. [DOI] [PubMed] [Google Scholar]
- 202.Plum P.S., Ulase D., Bollschweiler E., et al. Upregulation of insulin-like growth factor II mRNA-binding protein 3 (IMP3) has negative prognostic impact on early invasive (pT1) adenocarcinoma of the esophagus. J Cancer Res Clin Oncol. 2018;144(9):1731–1739. doi: 10.1007/s00432-018-2698-1. [DOI] [PubMed] [Google Scholar]
- 203.Jeng Y.M., Chang C.C., Hu F.C., et al. RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology. 2008;48(4):1118–1127. doi: 10.1002/hep.22459. [DOI] [PubMed] [Google Scholar]
- 204.Jiang Z., Chu P.G., Woda B.A., et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006;7(7):556–564. doi: 10.1016/S1470-2045(06)70732-X. [DOI] [PubMed] [Google Scholar]
- 205.Pei X., Li M., Zhan J., et al. Enhanced IMP3 expression activates NF-кB pathway and promotes renal cell carcinoma progression. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 206.Bell J.L., Turlapati R., Liu T., Schulte J.H., Hüttelmaier S. IGF2BP1 harbors prognostic significance by gene gain and diverse expression in neuroblastoma. J Clin Oncol. 2015;33(11):1285–1293. doi: 10.1200/JCO.2014.55.9880. [DOI] [PubMed] [Google Scholar]
- 207.Fan L., Lin Y., Lei H., et al. A newly defined risk signature, consisting of three m6A RNA methylation regulators, predicts the prognosis of ovarian cancer. Aging (Albany NY) 2020;12(18):18453–18475. doi: 10.18632/aging.103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li N., Zhan X. Identification of pathology-specific regulators of m6A RNA modification to optimize lung cancer management in the context of predictive, preventive, and personalized medicine. EPMA J. 2020;11(3):485–504. doi: 10.1007/s13167-020-00220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang Y., Ren F., Song Z., Wang X., Ma X. Multiomics profile and prognostic gene signature of m6A regulators in uterine corpus endometrial carcinoma. J Cancer. 2020;11(21):6390–6401. doi: 10.7150/jca.46386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang X., Fu X., Zhang J., Xiong C., Zhang S., Lv Y. Identification and validation of m6A RNA methylation regulators with clinical prognostic value in Papillary thyroid cancer. Cancer Cell Int. 2020;20:203. doi: 10.1186/s12935-020-01283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Johnson R.M., Phillips H.S., Bais C., et al. Development of a gene expression-based prognostic signature for IDH wild-type glioblastoma. Neuro Oncol. 2020;22(12):1742–1756. doi: 10.1093/neuonc/noaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Niedworok C., Panitz M., Szarvas T., et al. Urachal carcinoma of the bladder: impact of clinical and immunohistochemical parameters on prognosis. J Urol. 2016;195(6):1690–1696. doi: 10.1016/j.juro.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 213.Chromecki T.F., Cha E.K., Pummer K., et al. Prognostic value of insulin-like growth factor II mRNA binding protein 3 in patients treated with radical prostatectomy. BJU Int. 2012;110(1):63–68. doi: 10.1111/j.1464-410X.2011.10703.x. [DOI] [PubMed] [Google Scholar]
- 214.Ikenberg K., Fritzsche F.R., Zuerrer-Haerdi U., et al. Insulin-like growth factor II mRNA binding protein 3 (IMP3) is overexpressed in prostate cancer and correlates with higher Gleason scores. BMC Cancer. 2010;10:341. doi: 10.1186/1471-2407-10-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang Z., Cheng H., Xu H., Yu X., Sui D. A five-gene signature derived from m6A regulators to improve prognosis prediction of neuroblastoma. Cancer Biomarkers. 2020;28(3):275–284. doi: 10.3233/CBM-191196. [DOI] [PubMed] [Google Scholar]
- 216.Ma Y., Yin S., Liu X.F., et al. Comprehensive analysis of the functions and prognostic value of RNA-binding proteins in thyroid cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.625007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hou M., Guo X., Chen Y., Cong L., Pan C. A prognostic molecular signature of N⁶-methyladenosine methylation regulators for soft-tissue sarcoma from the cancer genome atlas database. Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.928400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mizukami Y., Kono K., Daigo Y., et al. Detection of novel cancer-testis antigen-specific T-cell responses in TIL, regional lymph nodes, and PBL in patients with esophageal squamous cell carcinoma. Cancer Sci. 2008;99(7):1448–1454. doi: 10.1111/j.1349-7006.2008.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang Z., Zhang C., Luo Y., et al. m6A regulator expression profile predicts the prognosis, benefit of adjuvant chemotherapy, and response to anti-PD-1 immunotherapy in patients with small-cell lung cancer. BMC Med. 2021;19(1):284. doi: 10.1186/s12916-021-02148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Akazawa Y., Hosono A., Yoshikawa T., et al. Efficacy of the NCCV Cocktail-1 vaccine for refractory pediatric solid tumors: A phase I clinical trial. Cancer Sci. 2019;110(12):3650–3662. doi: 10.1111/cas.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dahlem C., Abuhaliema A., Kessler S.M., et al. First small-molecule inhibitors targeting the RNA-binding protein IGF2BP2/IMP2 for cancer therapy. ACS Chem Biol. 2022;17(2):361–375. doi: 10.1021/acschembio.1c00833. [DOI] [PubMed] [Google Scholar]
- 222.Wallis N., Oberman F., Shurrush K., et al. Small molecule inhibitor of Igf2bp1 represses Kras and a pro-oncogenic phenotype in cancer cells. RNA Biol. 2022;19(1):26–43. doi: 10.1080/15476286.2021.2010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mahapatra L., Andruska N., Mao C., Le J., Shapiro D.J. A novel IMP1 inhibitor, BTYNB, targets c-myc and inhibits melanoma and ovarian cancer cell proliferation. Transl Oncol. 2017;10(5):818–827. doi: 10.1016/j.tranon.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yaqoob A., Li W.M., Liu V., et al. Grifolin, neogrifolin and confluentin from the terricolous polypore Albatrellus flettii suppress KRAS expression in human colon cancer cells. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0231948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang S.S., Lv Y., Xu X.C., et al. Triptonide inhibits human nasopharyngeal carcinoma cell growth via disrupting Lnc-RNA THOR-IGF2BP1 signaling. Cancer Lett. 2019;443:13–24. doi: 10.1016/j.canlet.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 226.Zhang Y., Liu X., Yu M., et al. Berberine inhibits proliferation and induces G0/G1 phase arrest in colorectal cancer cells by downregulating IGF2BP3. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118413. [DOI] [PubMed] [Google Scholar]
- 227.Goga A., Stoffel M. Therapeutic RNA-silencing oligonucleotides in metabolic diseases. Nat Rev Drug Discov. 2022;21(6):417–439. doi: 10.1038/s41573-022-00407-5. [DOI] [PubMed] [Google Scholar]
- 228.Mehmood K., Akhtar D., Mackedenski S., Wang C., Lee C.H. Inhibition of GLI1 expression by targeting the CRD-BP-GLI1 mRNA interaction using a specific oligonucleotide. Mol Pharmacol. 2016;89(6):606–617. doi: 10.1124/mol.115.102434. [DOI] [PubMed] [Google Scholar]
- 229.King D.T., Barnes M., Thomsen D., Lee C.H. Assessing specific oligonucleotides and small molecule antibiotics for the ability to inhibit the CRD-BP-CD44 RNA interaction. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang W., Goswami S., Lapidus K., et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64(23):8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 231.Cheng J., Demeulemeester J., Wedge D.C., et al. Pan-cancer analysis of homozygous deletions in primary tumours uncovers rare tumour suppressors. Nat Commun. 2017;8(1):1221. doi: 10.1038/s41467-017-01355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fawzy I.O., Hamza M.T., Hosny K.A., Esmat G., Abdelaziz A.I. Abrogating the interplay between IGF2BP1, 2 and 3 and IGF1R by let-7i arrests hepatocellular carcinoma growth. Growth Factors. 2016;34(1–2):42–50. doi: 10.3109/08977194.2016.1169532. [DOI] [PubMed] [Google Scholar]
- 233.Ko C.Y., Wang W.L., Li C.F., et al. IL-18-induced interaction between IMP3 and HuR contributes to COX-2 mRNA stabilization in acute myeloid leukemia. J Leukoc Biol. 2016;99(1):131–141. doi: 10.1189/jlb.2A0414-228RR. [DOI] [PubMed] [Google Scholar]
- 234.He H., Wu W., Sun Z., Chai L. miR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m6A-caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517(4):581–587. doi: 10.1016/j.bbrc.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 235.Gutschner T., Hämmerle M., Pazaitis N., et al. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) is an important protumorigenic factor in hepatocellular carcinoma. Hepatology. 2014;59(5):1900–1911. doi: 10.1002/hep.26997. [DOI] [PubMed] [Google Scholar]
- 236.Hsieh Y.T., Chou M.M., Chen H.C., Tseng J.J. IMP1 promotes choriocarcinoma cell migration and invasion through the novel effectors RSK2 and PPME1. Gynecol Oncol. 2013;131(1):182–190. doi: 10.1016/j.ygyno.2013.07.106. [DOI] [PubMed] [Google Scholar]
- 237.Stöhr N., Hüttelmaier S. IGF2BP1: a post-transcriptional "driver" of tumor cell migration. Cell Adhes Migrat. 2012;6(4):312–318. doi: 10.4161/cam.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yu D., Pan M., Li Y., et al. RNA N6-methyladenosine reader IGF2BP2 promotes lymphatic metastasis and epithelial-mesenchymal transition of head and neck squamous carcinoma cells via stabilizing slug mRNA in an m6A-dependent manner. J Exp Clin Cancer Res. 2022;41(1):6. doi: 10.1186/s13046-021-02212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sa R., Liang R., Qiu X., He Z., Liu Z., Chen L. Targeting IGF2BP2 promotes differentiation of radioiodine refractory papillary thyroid cancer via destabilizing RUNX2 mRNA. Cancers. 2022;14(5):1268. doi: 10.3390/cancers14051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang S., Gan M., Chen C., et al. Methyl CpG binding protein 2 promotes colorectal cancer metastasis by regulating N6-methyladenosine methylation through methyltransferase-like 14. Cancer Sci. 2021;112(8):3243–3254. doi: 10.1111/cas.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wu H., Ding X., Hu X., et al. LINC01021 maintains tumorigenicity by enhancing N6-methyladenosine reader IMP2 dependent stabilization of MSX1 and JARID2:implication in colorectal cancer. Oncogene. 2022;41(13):1959–1973. doi: 10.1038/s41388-022-02189-x. [DOI] [PubMed] [Google Scholar]
- 242.Li Z., Zhang Y., Ramanujan K., Ma Y., Kirsch D.G., Glass D.J. Oncogenic NRAS, required for pathogenesis of embryonic rhabdomyosarcoma, relies upon the HMGA2-IGF2BP2 pathway. Cancer Res. 2013;73(10):3041–3050. doi: 10.1158/0008-5472.CAN-12-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Samanta S., Pursell B., Mercurio A.M. IMP3 protein promotes chemoresistance in breast cancer cells by regulating breast cancer resistance protein (ABCG2) expression. J Biol Chem. 2013;288(18):12569–12573. doi: 10.1074/jbc.C112.442319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Qian L.X., Cao X., Du M.Y., et al. KIF18A knockdown reduces proliferation, migration, invasion and enhances radiosensitivity of esophageal cancer. Biochem Biophys Res Commun. 2021;557:192–198. doi: 10.1016/j.bbrc.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 245.Li K., Huang F., Li Y., et al. Stabilization of oncogenic transcripts by the IGF2BP3/ELAVL1 complex promotes tumorigenicity in colorectal cancer. Am J Cancer Res. 2020;10(8):2480–2494. [PMC free article] [PubMed] [Google Scholar]
- 246.Yang Z., Zhao F., Gu X., et al. Binding of RNA m6A by IGF2BP3 triggers chemoresistance of HCT8 cells via upregulation of ABCB1. Am J Cancer Res. 2021;11(4):1428–1445. [PMC free article] [PubMed] [Google Scholar]
- 247.Palanichamy J.K., Tran T.M., Howard J.M., et al. RNA-binding protein IGF2BP3 targeting of oncogenic transcripts promotes hematopoietic progenitor proliferation. J Clin Invest. 2016;126(4):1495–1511. doi: 10.1172/JCI80046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang N., Shen Y., Li H., et al. The m6A reader IGF2BP3 promotes acute myeloid leukemia progression by enhancing RCC2 stability. Exp Mol Med. 2022;54(2):194–205. doi: 10.1038/s12276-022-00735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mancarella C., Caldoni G., Ribolsi I., et al. Insulin-like growth factor 2 mRNA-binding protein 3 modulates aggressiveness of ewing sarcoma by regulating the CD164-CXCR4 axis. Front Oncol. 2020;10:994. doi: 10.3389/fonc.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gao Y., Luo T., Ouyang X., Zhu C., Zhu J., Qin X. IGF2BP3 and miR 191-5p synergistically increase HCC cell invasiveness by altering ZO-1 expression. Oncol Lett. 2020;20(2):1423–1431. doi: 10.3892/ol.2020.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Slosar M., Vohra P., Prasad M., Fischer A., Quinlan R., Khan A. Insulin-like growth factor mRNA binding protein 3 (IMP3) is differentially expressed in benign and malignant follicular patterned thyroid tumors. Endocr Pathol. 2009;20(3):149–157. doi: 10.1007/s12022-009-9079-x. [DOI] [PubMed] [Google Scholar]
- 252.Lu D., Vohra P., Chu P.G., Woda B., Rock K.L., Jiang Z. An oncofetal protein IMP3: a new molecular marker for the detection of esophageal adenocarcinoma and high-grade dysplasia. Am J Surg Pathol. 2009;33(4):521–525. doi: 10.1097/PAS.0b013e31818aada9. [DOI] [PubMed] [Google Scholar]
- 253.Kazeminezhad B., Mirafsharieh S.A., Dinyari K., Azizi D., Ebrahimi A. Usefulness of insulin-like growth factor II mRNA-binding protein 3 (IMP3) as a new marker for the diagnosis of esophageal adenocarcinoma in challenging cases. Turk J Gastroenterol. 2014;25(3):253–256. doi: 10.5152/tjg.2014.5454. [DOI] [PubMed] [Google Scholar]
- 254.Simon R., Bourne P.A., Yang Q., et al. Extrapulmonary small cell carcinomas express K homology domain containing protein overexpressed in cancer, but carcinoid tumors do not. Hum Pathol. 2007;38(8):1178–1183. doi: 10.1016/j.humpath.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 255.Yantiss R.K., Woda B.A., Fanger G.R., et al. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol. 2005;29(2):188–195. doi: 10.1097/01.pas.0000149688.98333.54. [DOI] [PubMed] [Google Scholar]
- 256.Nischalke H.D., Schmitz V., Luda C., et al. Detection of IGF2BP3, HOXB7, and NEK2 mRNA expression in brush cytology specimens as a new diagnostic tool in patients with biliary strictures. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sasaki M., Sato Y. An immunohistochemical panel of insulin-like growth factor II mRNA-binding protein 3 (IMP3), enhancer of zeste homolog 2 (EZH2), and p53 is useful for a diagnosis in bile duct biopsy. Virchows Arch. 2021;479(4):697–703. doi: 10.1007/s00428-021-03132-3. [DOI] [PubMed] [Google Scholar]
- 258.Sasaki M., Sato Y. Insulin-like growth factor II mRNA-binding protein 3 (IMP3) is a marker that predicts presence of invasion in papillary biliary tumors. Hum Pathol. 2017;62:152–159. doi: 10.1016/j.humpath.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 259.Kawashima H., Itoh A., Ohno E., et al. Diagnostic and prognostic value of immunohistochemical expression of S100P and IMP3 in transpapillary biliary forceps biopsy samples of extrahepatic bile duct carcinoma. J Hepatobiliary Pancreat Sci. 2013;20(4):441–447. doi: 10.1007/s00534-012-0581-z. [DOI] [PubMed] [Google Scholar]
- 260.Wang Y., Wang Y., Li D., et al. IMP3 signatures of fallopian tube: a risk for pelvic serous cancers. J Hematol Oncol. 2014;7:49. doi: 10.1186/s13045-014-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Visser N.C.M., van der Putten L.J.M., van Egerschot A., et al. Addition of IMP3 to L1CAM for discrimination between low- and high-grade endometrial carcinomas: a European Network for Individualised Treatment of Endometrial Cancer collaboration study. Hum Pathol. 2019;89:90–98. doi: 10.1016/j.humpath.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 262.Goodman S., Zhang L., Cheng L., Jiang Z. Differential expression of IMP3 between male and female mature teratomas: immunohistochemical evidence of malignant nature. Histopathology. 2014;65(4):483–489. doi: 10.1111/his.12409. [DOI] [PubMed] [Google Scholar]
- 263.Semjén D., Bíró K., Kapitány E., Kálmán E., Tornóczky T., Kajtár B. Histology, 12p status, and IMP3 expression separate subtypes in testicular teratomas. Virchows Arch. 2020;477(1):103–110. doi: 10.1007/s00428-020-02771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang Y., Li L., Wang Y., et al. IMP3 as a cytoplasmic biomarker for early serous tubal carcinogenesis. J Exp Clin Cancer Res. 2014;33(1):60. doi: 10.1186/s13046-014-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sharma G., Boby E., Nidhi T., et al. Diagnostic utility of IGF2BP1 and its targets as potential biomarkers in ETV6-RUNX1 positive B-cell acute lymphoblastic leukemia. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Okabayshi M., Kataoka T.R., Oji M., et al. IGF2BP3 (IMP3) expression in angiosarcoma, epithelioid hemangioendothelioma, and benign vascular lesions. Diagn Pathol. 2020;15(1):26. doi: 10.1186/s13000-020-00951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tang H., Wei Q., Ge J., et al. IMP3 as a supplemental diagnostic marker for Hodgkin lymphoma. Hum Pathol. 2013;44(10):2167–2172. doi: 10.1016/j.humpath.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 268.Masoud R., Ibrahiem A., Tantawy D., Eldosoky I. The complementary role of insulin-like growth factor II mRNA-binding protein 3 (IMP3) in diagnosis of Hodgkin's lymphoma. Ann Diagn Pathol. 2019;42:64–68. doi: 10.1016/j.anndiagpath.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 269.Cornejo K., Shi M., Jiang Z. Oncofetal protein IMP3: a useful diagnostic biomarker for leiomyosarcoma. Hum Pathol. 2012;43(10):1567–1572. doi: 10.1016/j.humpath.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 270.Shooshtarizadeh T., Nazeri A., Zare-Mirzaie A., Movahedinia S. Expression of insulin-like growth factor ІI mRNA binding protein 3 (IMP3) in enchondroma and chondrosarcoma. Pathol Res Pract. 2016;212(4):335–339. doi: 10.1016/j.prp.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 271.Laban S., Gangkofner D.S., Holzinger D., et al. Antibody responses to cancer antigens identify patients with a poor prognosis among HPV-positive and HPV-negative head and neck squamous cell carcinoma patients. Clin Cancer Res. 2019;25(24):7405–7412. doi: 10.1158/1078-0432.CCR-19-1490. [DOI] [PubMed] [Google Scholar]
- 272.Deng X., Jiang Q., Liu Z., Chen W. Clinical significance of an m6A reader gene, IGF2BP2, in head and neck squamous cell carcinoma. Front Mol Biosci. 2020;7:68. doi: 10.3389/fmolb.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lin C.Y., Chen S.T., Jeng Y.M., et al. Insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor formation and invasion and predicts poor prognosis in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40(9):699–705. doi: 10.1111/j.1600-0714.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 274.Li H.G., Han J.J., Huang Z.Q., Wang L., Chen W.L., Shen X.M. IMP3 is a novel biomarker to predict metastasis and prognosis of tongue squamous cell carcinoma. J Craniofac Surg. 2011;22(6):2022–2025. doi: 10.1097/SCS.0b013e3182319750. [DOI] [PubMed] [Google Scholar]
- 275.Asioli S., Erickson L.A., Righi A., et al. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol. 2010;23(9):1269–1278. doi: 10.1038/modpathol.2010.117. [DOI] [PubMed] [Google Scholar]
- 276.Li Y., Gu J., Xu F., et al. Molecular characterization, biological function, tumor microenvironment association and clinical significance of m6A regulators in lung adenocarcinoma. Briefings Bioinf. 2021;22(4):bbaa225. doi: 10.1093/bib/bbaa225. [DOI] [PubMed] [Google Scholar]
- 277.Guo W., Huai Q., Wan H., et al. Prognostic impact of IGF2BP3 expression in patients with surgically resected lung adenocarcinoma. DNA Cell Biol. 2021;40(2):316–331. doi: 10.1089/dna.2020.6136. [DOI] [PubMed] [Google Scholar]
- 278.Zhong S., Lin Z., Chen H., Mao L., Feng J., Zhou S. The m6A-related gene signature for predicting the prognosis of breast cancer. PeerJ. 2021;9 doi: 10.7717/peerj.11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yang L., Wu S., Ma C., et al. RNA m6A methylation regulators subclassify luminal subtype in breast cancer. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.611191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Walter O., Prasad M., Lu S., Quinlan R.M., Edmiston K.L., Khan A. IMP3 is a novel biomarker for triple negative invasive mammary carcinoma associated with a more aggressive phenotype. Hum Pathol. 2009;40(11):1528–1533. doi: 10.1016/j.humpath.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 281.Takizawa K., Yamamoto H., Taguchi K., et al. Insulin-like growth factor II messenger RNA-binding protein-3 is an indicator of malignant phyllodes tumor of the breast. Hum Pathol. 2016;55:30–38. doi: 10.1016/j.humpath.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 282.Ohashi R., Sangen M., Namimatsu S., Takei H., Naito Z. IMP3 contributes to poor prognosis of patients with metaplastic breast carcinoma: a clinicopathological study. Ann Diagn Pathol. 2017;31:30–35. doi: 10.1016/j.anndiagpath.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 283.Wakita A., Motoyama S., Sato Y., et al. IGF2BP3 expression correlates with poor prognosis in esophageal squamous cell carcinoma. J Surg Res. 2021;259:137–144. doi: 10.1016/j.jss.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 284.Schaeffer D.F., Owen D.R., Lim H.J., et al. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) overexpression in pancreatic ductal adenocarcinoma correlates with poor survival. BMC Cancer. 2010;10:59. doi: 10.1186/1471-2407-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Okada K., Fujiwara Y., Nakamura Y., et al. Oncofetal protein, IMP-3, a potential marker for prediction of postoperative peritoneal dissemination in gastric adenocarcinoma. J Surg Oncol. 2012;105(8):780–785. doi: 10.1002/jso.22108. [DOI] [PubMed] [Google Scholar]
- 286.Liu X., Chen Z., Zhao X., et al. Effects of IGF2BP2, KCNQ1 and GCKR polymorphisms on clinical outcome in metastatic gastric cancer treated with EOF regimen. Pharmacogenomics. 2015;16(9):959–970. doi: 10.2217/pgs.15.49. [DOI] [PubMed] [Google Scholar]
- 287.Kim S.J., Kim S.W., Oh C.H., et al. Expression of insulin-like growth factor II mRNA-binding protein 3 in gallbladder carcinoma. Anticancer Res. 2020;40(10):5777–5785. doi: 10.21873/anticanres.14594. [DOI] [PubMed] [Google Scholar]
- 288.Kim H.G., Park M.S., Sung J.Y., Kim Y.W., Kim H.S., Na K. Tumor-specific expression of insulin-like growth factor II mRNA-binding protein 3 independently predicts worse survival of patients with adenocarcinoma of the Ampulla of vater. Anticancer Res. 2019;39(9):4947–4955. doi: 10.21873/anticanres.13683. [DOI] [PubMed] [Google Scholar]
- 289.Liu H., Zeng Z., Afsharpad M., et al. Overexpression of IGF2BP3 as a potential oncogene in ovarian clear cell carcinoma. Front Oncol. 2019;9:1570. doi: 10.3389/fonc.2019.01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zou Z., Zhou S., Liang G., et al. The pan-cancer analysis of the two types of uterine cancer uncovered clinical and prognostic associations with m6A RNA methylation regulators. Mol Omics. 2021;17(3):438–453. doi: 10.1039/d0mo00113a. [DOI] [PubMed] [Google Scholar]
- 291.Cong R., Ji C., Zhang J., et al. m6A RNA methylation regulators play an important role in the prognosis of patients with testicular germ cell tumor. Transl Androl Urol. 2021;10(2):662–679. doi: 10.21037/tau-20-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Szarvas T., Tschirdewahn S., Niedworok C., et al. Prognostic value of tissue and circulating levels of IMP3 in prostate cancer. Int J Cancer. 2014;135(7):1596–1604. doi: 10.1002/ijc.28808. [DOI] [PubMed] [Google Scholar]
- 293.Xing Q.W., Luan J.C., Liu S.Y., Ma L.M., Wang Y. Six RNA binding proteins (RBPs) related prognostic model predicts overall survival for clear cell renal cell carcinoma and is associated with immune infiltration. Bosn J Basic Med Sci. 2022;22(3):435–452. doi: 10.17305/bjbms.2021.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Jiang Z., Lohse C.M., Chu P.G., et al. Oncofetal protein IMP3: a novel molecular marker that predicts metastasis of papillary and chromophobe renal cell carcinomas. Cancer. 2008;112(12):2676–2682. doi: 10.1002/cncr.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Huang W., Li Y., Zhang C., et al. IGF2BP3 facilitates cell proliferation and tumorigenesis via modulation of JAK/STAT signalling pathway in human bladder cancer. J Cell Mol Med. 2020;24(23):13949–13960. doi: 10.1111/jcmm.16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sitnikova L., Mendese G., Liu Q., et al. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res. 2008;14(6):1701–1706. doi: 10.1158/1078-0432.CCR-07-2039. [DOI] [PubMed] [Google Scholar]
- 297.Lee D.J., Xylinas E., Rieken M., et al. Insulin-like growth factor messenger RNA-binding protein 3 expression helps prognostication in patients with upper tract urothelial carcinoma. Eur Urol. 2014;66(2):379–385. doi: 10.1016/j.eururo.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 298.Chen S.T., Jeng Y.M., Chang C.C., et al. Insulin-like growth factor II mRNA-binding protein 3 expression predicts unfavorable prognosis in patients with neuroblastoma. Cancer Sci. 2011;102(12):2191–2198. doi: 10.1111/j.1349-7006.2011.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhang X., Zhong L., Zou Z., et al. Clinical and prognostic pan-cancer analysis of N6-methyladenosine regulators in two types of hematological malignancies: a retrospective study based on TCGA and GTEx databases. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.623170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Mäkinen A., Nikkilä A., Haapaniemi T., et al. IGF2BP3 associates with proliferative phenotype and prognostic features in B-cell acute lymphoblastic leukemia. Cancers. 2021;13(7):1505. doi: 10.3390/cancers13071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sheen Y.S., Liao Y.H., Lin M.H., et al. IMP-3 promotes migration and invasion of melanoma cells by modulating the expression of HMGA2 and predicts poor prognosis in melanoma. J Invest Dermatol. 2015;135(4):1065–1073. doi: 10.1038/jid.2014.480. [DOI] [PubMed] [Google Scholar]
- 302.Del Gobbo A., Vaira V., Guerini Rocco E., et al. The oncofetal protein IMP3: a useful marker to predict poor clinical outcome in neuroendocrine tumors of the lung. J Thorac Oncol. 2014;9(11):1656–1661. doi: 10.1097/JTO.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 303.Wang L., Aireti A., Aihaiti A., Li K. Expression of microRNA-150 and its target gene IGF2BP1 in human osteosarcoma and their clinical implications. Pathol Oncol Res. 2019;25(2):527–533. doi: 10.1007/s12253-018-0454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Liu H., Qin G., Ji Y., et al. Potential role of m6A RNA methylation regulators in osteosarcoma and its clinical prognostic value. J Orthop Surg Res. 2021;16(1):294. doi: 10.1186/s13018-021-02422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yasutake N., Ohishi Y., Taguchi K., et al. Insulin-like growth factor II messenger RNA-binding protein-3 is an independent prognostic factor in uterine leiomyosarcoma. Histopathology. 2018;72(5):739–748. doi: 10.1111/his.13422. [DOI] [PubMed] [Google Scholar]