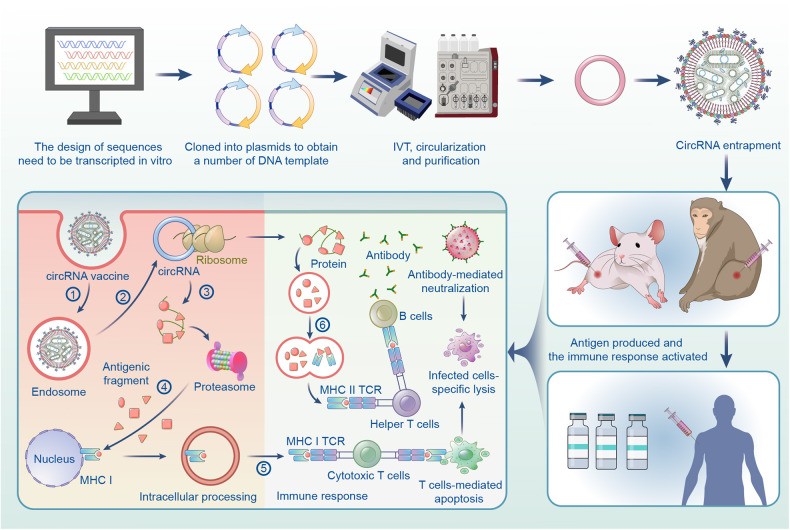
Fig. 2.
Establishment of circRNA vaccine production pipeline and circRNA vaccines to elicit immunity by utilizing disease-specific targeted antigen strategies. Briefly, The encoding sequence of peptide/protein is designed according to the intended antigen and cloned into a plasmid DNA construct. Plasmid DNA is transcripted into linear RNA precursor (pre-circRNA) by in vitro transcription (IVT) technology. Then the pre-circRNA is cyclized as circRNA in vitro. And circRNA is further purified by high-performance liquid chromatography (HPLC) to remove contaminants and reactants. Subsequently, purified circRNA is entrapped in various vehicles. Prior to clinical trials, pharmacodynamic and biosafety evaluation should be conducted. Finally, the scale-up manufacturing of circRNA vaccine is followed by the clinical trial. In the induction of antigen-specific immune responses. The circRNA vaccines mainly follow three aspects in intracellular processes, including endosome escape, antigen encoding, and immune initiation. Briefly, In antigen-presenting cells (APCs), (1) LNPs containing circRNA vaccines form endosomes in the cytoplasm. Then, (2) endosomes release the circRNA vaccine (endosome escape). Subsequently, (3) encoding sequences in circRNA were translated into antigenic proteins/peptides by ribosomes. (4) endogenous antigens are degraded into polypeptides by the proteasome and are presented by MHC I and (5) activate cytotoxic T cells (CD8+ T cells). In addition to cellular immunity, circRNA-induced humoral immunity is also important for disease prevention. (6) endogenous antigens in APCs can be secreted and be presented to the helper T cells by MHC class II proteins. Helper T cells (CD4+ T cells) stimulate B cells to produce neutralizing antibodies