Abstract
Background
Maternal obesity has been associated with shorter breastfeeding duration, but little is known about mediating factors explaining this association. It is important to assess these relationships across diverse populations because breastfeeding is culturally patterned.
Objectives
We investigated the association of prepregnancy maternal body mass index (BMI) with breastfeeding outcomes and potential mediators of this relationship in 3 culturally diverse international cohorts.
Methods
We analyzed 5120 singleton pregnancies from mother–child cohorts in Spain (INfancia y Medio Ambiente), Greece (Rhea), and the United States (Project Viva). Outcome variables were duration of any and exclusive breastfeeding. A priori hypothesized mediators in the association of maternal prepregnancy BMI with breastfeeding were birthweight (BW), maternal prenatal C-reactive protein (CRP), cesarean delivery, maternal dietary inflammatory index (DII) during pregnancy, gestational age at delivery, and gestational diabetes mellitus (GDM). We estimated the association between BMI and breastfeeding duration using linear regression adjusting for confounders. Mediation analysis estimated direct and indirect effects of maternal overweight/obesity on breastfeeding for each mediator.
Results
Women with overweight and obesity had shorter duration of any and exclusive breastfeeding compared with normal-weight women (any: overweight β = −0.79 mo, 95% CI: −1.17, −0.40; obese β = −1.75 mo 95% CI: −2.25, −1.25; exclusive: overweight β = −0.30 mo, 95% CI: −0.42, −0.16; obese β = −0.73 mo, 95% CI: −0.90, −0.55). Significant mediators (% change in effect estimate) of this association were higher CRP (exclusive: 5.12%), cesarean delivery (any: 6.54%; exclusive: 7.69%), and higher DII (any: 6.48%; exclusive: 7.69%). GDM, gestational age, and BW did not mediate the association of maternal weight status with breastfeeding.
Conclusions
Higher prepregnancy BMI is associated with shorter duration of any and exclusive breastfeeding. Maternal dietary inflammation, systemic inflammation, and mode of delivery may be key modifiable mediators of this association. Identification of mediators provides potential targets for interventions to improve breastfeeding outcomes.
Keywords: maternal obesity, maternal metabolism, breastfeeding duration, lactation outcomes
Introduction
Exclusive breastfeeding is recommended for at least 6 mo and any breastfeeding for up to 2 y, because of the durable health benefits for mothers and children [1,2]. However, breastfeeding rates and duration of exclusive breastfeeding in the United States [3] and Europe [4] continue to fall below national and international standards. Although recent public health efforts have led to some improvement in breastfeeding rates, women with overweight and obesity, who constitute >60% of women of childbearing age [5,6], continue to be at higher risk for suboptimal breastfeeding.
The association between maternal obesity and shorter breastfeeding duration has been reported since at least 1992 [7]. Several more recent retrospective and prospective single-nation studies similarly found decreased breastfeeding initiation and duration in women with obesity [[8], [9], [10], [11]]. Although epidemiologic studies have demonstrated the association between maternal BMI and breastfeeding outcomes, including some investigation into mediators such as gestational weight gain, mode of delivery, gestational diabetes mellitus (GDM), infant weight, and sociodemographic characteristics, the underlying factors explaining this association remain unclear. Animal and small human studies have suggested the pathogenic role of inflammation in shorter breastfeeding durations [12], but this has not been examined in larger human cohorts. CRP is a nonspecific marker of systemic inflammation that is associated with multiple proinflammatory stimuli, including greater dietary inflammation [13] and obesity [14,15]. Our group recently reported that a higher maternal dietary inflammatory index (DII), indicating higher dietary inflammation [13], predicted poor breastfeeding outcomes in Project Viva, a United States-based cohort [16]. Insulin resistance and GDM have also been postulated as potential mediators in this association, because women with obesity are more likely to be diagnosed with GDM, which is an independent risk factor for shorter breastfeeding duration [17]. In addition, gestational age [18], increased infant birthweight (BW) [19,20], and need for cesarean delivery [21] are all influenced by maternal BMI and have been shown to affect breastfeeding outcomes. One study that investigated the role of infant BW found that higher infant BW lessened the negative association between maternal BMI and breastfeeding outcomes [20]. Regardless of this prior evidence, studies quantifying mediating contributions of multiple mediators in the association between BMI and breastfeeding are lacking.
The available literature examining associations between maternal BMI and lactation outcomes is limited in that 1) most are single-nation cohorts, and obesity, breastfeeding, diet, and maternity practices are all closely linked to cultural and social contexts; and 2) comprehensive understanding of mediators in this association to inform mechanistic understanding and interventions is incomplete. To address this evidence gap, we conducted a pooled analysis of 3 international mother–infant cohorts with a detailed assessment of potential mediators, to examine the association of maternal BMI and breastfeeding duration, and identify key mediators of this association. We hypothesized that higher maternal BMI would be associated with shorter duration of any and exclusive breastfeeding, and that infant BW, maternal CRP, cesarean delivery, DII, gestational age, and GDM would mediate this association.
Methods
Study population and design
We analyzed data collected from 3 prospective cohort studies, pooling data from INfancia y Medio Ambiente (INMA) (Environment and Childhood Project) (n = 2764) in Spain, Rhea (n = 1252) in Greece, and Project Viva (n = 2128) in the United States. The INMA study population comprised 4 subcohorts from Spain based in the regions of Valencia, Sabadell, Asturias, and Gipuzkoa, recruited at the first prenatal visit (10–13 wk of gestation) during 2003–2008 [22]. The Rhea cohort recruited at the time of the first ultrasound examination at approximately 12 wk of gestation, during 2007–2008 in Crete, Greece [23]. Project Viva recruited pregnant women during their first routine obstetric visit (median 9.9 wk of gestation) at Atrius Harvard Vanguard Medical Associates in eastern Massachusetts between 1999 and 2002 [24]. All studies were conducted in accordance with the ethical guidelines for research outlined in the Declaration of Helsinki and the protocol for the pooled analysis was approved by the Institutional Review Board at Brigham and Women’s Hospital (#2014P002147). Informed consent was obtained from all mothers.
In this analysis, we included 5120 singleton pregnancies with maternal prepregnancy BMI and breastfeeding outcome data available (83% of the 6144 pregnant women initially enrolled, Supplemental Table 1, Supplemental Figure 1), consisting of 2312 from INMA, 1103 from Rhea, and 1705 from Viva (Table 1).
TABLE 1.
Participant characteristics by BMI category
| n | Total |
<18.5 |
18.5 to <25 |
25 to <30 |
≥30 |
P value | |
|---|---|---|---|---|---|---|---|
| n = 5120 | n = 206 | n = 3315 | n = 1034 | n = 565 | |||
| Mean (SD) or n (%) | |||||||
| Age at delivery (y) | 5107 | 31.72 (4.80) | 29.95 (5.48) | 31.77 (4.63) | 31.99 (4.97) | 31.67 (4.99) | <0.001 |
| Education, college level and above | 5107 | 2673 (52.34) | 92 (44.88) | 1786 (54.06) | 523 (50.63) | 272 (48.14) | <0.001 |
| Race/ethnicity | 5098 | — | — | — | — | — | — |
| Caucasian | — | 4430 (86.90) | 177 (86.34) | 2951 (89.45) | 863 (83.62) | 439 (78.11) | <0.001 |
| Other | — | 668 (13.10) | 28 (13.66) | 348 (10.55) | 169 (16.38) | 123 (21.89) | <0.001 |
| Marital status, married/living with partner | 5107 | 4831 (94.60) | 184 (89.76) | 3138 (94.92) | 984 (95.35) | 525 (93.09) | 0.003 |
| Smoking during pregnancy | 4628 | 1234 (26.66) | 67 (34.90) | 780 (25.99) | 257 (27.55) | 130 (25.90) | 0.049 |
| Nulliparous | 5112 | 2619 (51.23) | 117 (57.07) | 1813 (54.76) | 462 (44.77) | 227 (40.25) | <0.001 |
| DII (units) | 4467 | −0.92 (2.29) | −0.94 (2.49) | −0.97 (2.25) | −0.86 (2.36) | −0.68 (2.39) | 0.077 |
| CRP (mg/dL) 25th–75th % range (median) | 2580 | 0.12–0.5 (0.27) | 0.12–0.33 (0.20) | 0.10–0.44 (0.22) | 0.16–0.60 (0.33) | 0.23–1.03 (0.43) | <0.001 |
| Gestational diabetes mellitus | 4783 | 271 (5.67) | 8 (4.30) | 133 (4.30) | 69 (7.17) | 61 (11.44) | <0.001 |
| Delivered by cesarean section | 5055 | 1343 (26.57) | 48 (23.65) | 778 (23.84) | 312 (30.38) | 205 (36.54) | <0.001 |
| Gestation length (wk) | 5107 | 39.27 (1.73) | 39.12 (1.74) | 39.30 (1.74) | 39.30 (1.63) | 39.10 (1.86) | 0.034 |
| Infant gender, female | 5120 | 2491 (48.65) | 104 (50.49) | 1638 (49.41) | 463 (44.78) | 286 (50.62) | 0.043 |
| Birthweight (g) | 5093 | 3318.88 (515.53) | 3164.22 (443.49) | 3289.04 (508.71) | 3406.52 (506.42) | 3389.90 (563.35) | <0.001 |
| BW/GA Z-score, intergrowth | 5106 | 0.23 (1.07) | −0.06 (1.01) | 0.15 (1.06) | 0.42 (1.03) | 0.46 (1.13) | <0.001 |
| Ever breastfed | 5093 | 4340 (85.22) | 180 (87.38) | 2881 (87.60) | 848 (82.01) | 431 (76.42) | <0.001 |
| Any-breastfeeding duration (mo) | 5093 | 5.57 (5.71) | 4.86 (4.82) | 5.95 (5.79) | 5.21 (5.65) | 4.20 (5.32) | <0.001 |
| Exclusive-breastfeeding duration (mo) | 4146 | 1.86 (2.04) | 1.68 (1.97) | 2.05 (2.06) | 1.68 (2.02) | 1.16 (1.75) | <0.001 |
| Breastfeeding category | 5093 | <0.001 | |||||
| None | 753 (14.78) | 26 (12.62) | 408 (12.40) | 186 (17.99) | 133 (23.58) | ||
| 0 to <6 mo | 2322 (45.59) | 115 (55.83) | 1480 (45.00) | 463 (44.78) | 264 (46.81) | ||
| 6 to <12 mo | 1218 (23.92) | 38 (18.45) | 840 (25.54) | 234 (22.63) | 106 (18.79) | ||
| ≥12 mo | 800 (15.71) | 27 (13.11) | 561 (17.06) | 151 (14.60) | 61 (10.82) | ||
Analysis performed using 1-factor ANOVA for continuous variables and Pearson’s Chi-squared test for categorical variables.
Abbreviations: BW, birthweight; DII, dietary inflammatory index; GA, gestational age.
Prepregnancy BMI and obesity classification
In all cohorts, mothers self-reported prepregnancy weight, and either self-reported height (Viva) or had height measured in early pregnancy (Rhea, INMA), from which BMI (kg/m2) was calculated (BMI = weight in kg divided by height in m2). We defined BMI categories per the WHO: underweight <18.5 kg/m2, normal weight 18.5 to <25 kg/m2, overweight 25 to <30 kg/m2, and obese ≥30 kg/m2 [25].
Breastfeeding duration
Outcome variables included duration of 1) any breastfeeding and 2) exclusive breastfeeding in months. The INMA cohorts collected breastfeeding data via interview-based questionnaires at birth, 6, and 14 mo postpartum. They defined any breastfeeding as any at-the-breast feeding and expressed-milk feeding, allowing for intake of other foods and liquids, and exclusive breastfeeding as intake of breastmilk only, not allowing for water or other liquids, but allowing for oral rehydration solution, drops, and syrups [26]. The Rhea cohort collected breastfeeding data via questionnaires at 6 and 18 mo postpartum. They defined any breastfeeding as any amount of breastmilk, allowing for solids and liquids, and exclusive breastfeeding as only breastmilk, including expressed, allowing for drops and syrups [27]. The Viva cohort collected breastfeeding information via interviews and questionnaires at 6 and 12 mo postpartum. They defined any breastfeeding as any amount of maternal breast milk, including at-the-breast and expressed breastfeeding, and exclusive breastfeeding as only breastmilk without supplemental formula or foods [28]. For the purposes of this analysis, we measured exclusive breastfeeding through 6 mo; durations of longer than 6 mo were censored and categorized as 6-mo durations. We chose this approach based on international recommendations for exclusive breastfeeding durations [29] and the typical introduction of solid foods by 6 mo of age.
Assessment of mediators and confounders
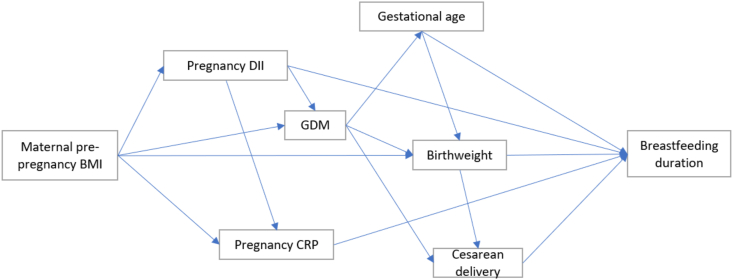
We selected potential mediators and confounders based on a priori knowledge using directed acyclic graphs [30]. Mediators between maternal prepregnancy BMI and breastfeeding outcomes included CRP [14], DII [16,31], GDM [17,32], cesarean delivery [21,33], gestational age [34,35], and BW [19,36] (Figure 1). Although recognizing that BMI and DII do have a bidirectional relationship [37], DII was used as a mediator in this analysis, given the biological plausibility that body habitus impacts dietary habits [31] and that dietary patterns and inflammation, in turn, impact breastfeeding [16].
FIGURE 1.
Simplified directed acyclic diagram of hypothesized mediating pathways and assumed interrelationships between mediators, based on a priori knowledge. DII, dietary inflammatory index; GDM, gestational diabetes mellitus.
CRP in the INMA cohort was measured in serum samples collected in the first pregnancy trimester. CRP measurement was conducted by immunoturbidimetry at the Consulting Químico Sanitario Laboratory for the Gipuzkoa subcohort and at the Echevarne Sabadell Laboratory for the Sabadell subcohort. CRP levels were not measured in the INMA-Valencia (n = 705) and Asturias (n =457) subcohorts [38]. The Rhea cohort measured CRP at a mean 14.1 wk of gestation (SD 4.5 wk) with a high-sensitivity homogeneous immunoassay [39]. Project Viva measured CRP between 22 and 31 wk of gestation [16].
Each cohort derived individual maternal DIIs from validated maternal food frequency questionairres (FFQs). The DII is a validated measure of inflammation in one’s diet, with a more negative value representing antiinflammatory effects of a diet, and more positive values being proinflammatory. In all 3 cohorts, mothers self-completed FFQs to assess dietary intake (Supplemental Methods). The scoring algorithm for DII was based on a literature search of articles that studied the effect of diet on inflammatory biomarkers, published from 1950 to 2010. Forty-five food parameters were included and were scored based on their effect on inflammation (whether they increased, decreased, or did not affect inflammatory markers). Intakes of nutrients were standardized based on a global database of studied populations around the world to provide a mean and standard deviation for each nutrient, and these were then converted to a proportion from 0 to 1. The proportions were then converted into food parameter-specific DII scores, which were subsequently added to result in the total DII score [40]. For the INMA cohort, FFQ data were collected in the first and third trimesters (12th and 32nd wk of gestation) [41]. For this analysis, we calculated the average DII from these timepoints as a better proxy for diet throughout pregnancy (Pearson’s r between 1st- and 3rd-trimester DII = 0.53, P < 0.0001). In the Rhea cohort, 1 FFQ was administered in the second trimester (14th—18th wk of gestation) [42]. For the Viva cohort, mothers completed FFQs in the first and second trimesters (median 9.9 and 27.9 wk gestation). We used the average DII score from these 2 timepoints, because they were highly correlated (Pearson’s r = 0.61, P < 0.0001) [16].
The diagnosis of GDM was obtained from medical records. Clinical diagnosis in all 3 geographical regions was based on a “2-step” screening and diagnostic approach. INMA participants were screened only if they were at high risk for GDM between 24 and 28 wk (based on age, BMI, family history of diabetes, and/or previous pregnancy complications), whereas in the Rhea and Viva cohorts, all pregnant women were screened at 24–28 wk of gestation [32,43,44]. The first step of the screening was a nonfasting 50-g oral glucose challenge test (GCT), followed by a 3-h 100-g oral glucose tolerance test (OGTT) for those with an abnormal blood glucose concentration of ≥140 mg/dL on the GCT. In the INMA cohort, the diagnosis of GDM was made if 2 or more OGTT glucose levels exceeded reference values of the National Diabetes Data Group (fasting ≥105 mg/dL, 1-h ≥190 mg/dL, 2-h ≥165 mg/dL, 3-h ≥145 mg/dL) [45]. In the Rhea and Viva cohorts, women were diagnosed with GDM if they had 2 or more abnormal glucose levels after the OGTT, per Carpenter/Coustan criteria (fasting ≥95 mg/dL, 1-h ≥180 mg/dL, 2-h ≥155 mg/dL, 3-h ≥140 mg/dL) [45].
Information on BW, cesarean delivery, and gestational age at birth was obtained from the birth hospital medical records. BW z-scores and categories of small for gestational age, appropriate for gestational age, and large for gestational age were generated using the Intergrowth-21st Growth Standard [46].
Confounders included maternal age at delivery (in y), education (less than college education compared with any college and above), parity, maternal marital status (married/cohabitating compared with not), smoking (any smoking compared with no smoking) during pregnancy, and race/ethnicity (Caucasian compared with other). Notably, we were limited in using Caucasian compared with others to classify maternal race/ethnicity because of available data collected across the cohorts. Confounders were self-reported by mothers at pregnancy visits.
Statistical analysis
We compared differences in variables across BMI categories using 1-factor ANOVA for continuous variables and Pearson’s Chi-squared test for categorical variables (Table 1).
We quantified the association of maternal BMI (modeled separately as both a continuous and categorical predictor) with a duration of any and exclusive breastfeeding using linear regression models, including unadjusted and multivariable-adjusted models. We used normal BMI as the reference group for the analysis by BMI category.
To test for heterogeneity among cohorts, a linear mixed-effect model was fitted with a fixed effect interaction term between BMI categories and cohorts, and a random intercept term for cohorts. We found little heterogeneity in associations between cohorts, and therefore all main analyses were performed in the pooled-cohort dataset with additional sensitivity analyses stratified by cohort.
We performed mediation analysis to estimate the total and direct effects of BMI category (<25 compared with ≥25 kg/m2) on the duration of any and exclusive breastfeeding and the indirect effects through hypothesized mediators (BW, CRP, cesarean delivery, DII, gestational age, and GDM) [47]. The proportion of the association mediated by each mediator was then calculated by dividing the indirect effect of the mediator by the total effect of BMI on the duration of breastfeeding. To reduce the likelihood for overadjustment collider-stratification bias [48,49] (Figure 1), mediation models considered each mediator separately.
To account for missing data and their inherent heterogeneity within cohorts, including missing CRP values, multiple imputation was conducted separately for each cohort using the Multiple Imputation by Chained Equations and results were compared with and without multiple imputation [50]. Sensitivity analysis was performed to investigate the effect of BMI on breastfeeding outcome stratified by infant gender and available sociodemographic variables, including maternal marital status and maternal education. To confirm the robustness of mediation results and test the strength of these associations in women with compared with without obesity, we repeated the analysis with a BMI exposure cut-off of 30 kg/m2.
All statistical analyses were performed using Stata Version 16.1. Mediation analyses were performed using the medeff command in Stata [47]. Any 2-sided P value <0.05 was considered statistically significant.
Results
Participant characteristics
In the combined cohort, 11.04% of women had obesity, 20.20% were overweight, and 64.75% normal BMI (Table 1). CRP, GDM, and cesarean delivery were all higher with higher BMI category. Of note, CRP had a large percentage of missing data (50.5%), in part because of the 2 INMA subcohorts that had not measured CRP. DII was higher in overweight and obese women compared with the normal BMI group and was also higher in the underweight group compared with normal-weight women. Infant BW z-score was higher with higher BMI categories. The percentage of women with college-level education was the highest in the normal BMI category at 54.06%, and lower with higher BMI category (50.63% and 48.14% in overweight and obese, respectively). When comparing characteristics by cohort (Supplemental Table 2), the Viva cohort had the highest mean prepregnancy BMI [Viva 24.83 kg/m2 (SD: 5.41), INMA 23.55 kg/m2 (SD: 4.23), Rhea 24.25 kg/m2 (SD: 4.77)]. The Viva cohort had the highest percentage of women with college-level education at 90.45%, compared with 34.62% in INMA and 33.62% in Rhea. Rhea’s population had the highest percentage of women diagnosed with GDM (9.28%), whereas INMA and Viva had 4.17% and 5.48% respectively. The mean DII was significantly higher in the Rhea cohort (2.55, SD: 1.37) than in INMA (−1.13, SD: 1.59) and Viva (−2.62, SD: 1.34).
Association of maternal obesity and breastfeeding duration
Higher prepregnancy BMI was significantly associated with shorter duration of any breastfeeding (β = −0.12 mo per 1 kg/m2 maternal BMI, 95% CI: −0.16, −0.09) and exclusive breastfeeding (β = −0.05 mo per 1 kg/m2 maternal BMI, 95% CI: −0.06, −0.04) in the unadjusted models. These associations were only slightly attenuated after adjusting for confounders (any breastfeeding: β = −0.11 mo per 1 kg/m2 maternal BMI, 95% CI: −0.15, −0.08; exclusive breastfeeding: β = −0.05 1 mo per 1 kg/m2 maternal BMI, 95% CI: −0.06, −0.04) (Supplemental Table 3).
Compared with women with normal BMI, the duration of any breastfeeding was 4 wk shorter for women with overweight (β = −0.79 mo, 95% CI: −1.17, −0.40) and 7.5 wk shorter for women with obesity (β = −1.75 mo, 95% CI: −2.25, −1.25) in fully adjusted models (Table 2, Supplemental Figure 2). Similarly, the duration of exclusive breastfeeding was shorter for women with overweight by 9 d (β = −0.30 mo, 95% CI: −0.42, −0.16) and obesity by 3 wk (β = −0.73 mo, 95% CI: −0.90, −0.55) compared with normal-weight women (Table 2). Among those with obesity, these associations were stronger with increasing obesity class (Supplemental Table 4).
TABLE 2.
Association of maternal BMI (kg/m2, categorical) with duration of breastfeeding (months), compared with normal BMI
| Any breastfeeding |
Exclusive breastfeeding |
|||
|---|---|---|---|---|
| Crude model β (95% CI)1 | Fully adjusted model β (95% CI)2 | Crude model β (95% CI)1 | Fully adjusted model β (95% CI)2 | |
| Total (n = 5120) | ||||
| Underweight | −0.983 (−1.77, −0.19) | −0.66 (−1.44, 0.12) | −0.353 (−0.63, −0.07) | −0.17 (−0.44, 0.10) |
| Overweight | −0.853 (−1.24, −0.46) | −0.793 (−1.17, −0.40) | −0.333 (−0.46, −0.19) | −0.303 (−0.42, −0.16) |
| Obese | −1.953 (−2.45, −1.45) | −1.753 (−2.25, −1.25) | −0.803 (−0.98, −0.62) | −0.733 (−0.90, −0.55) |
| INMA (n = 2312) | ||||
| Underweight | −0.29 (−1.33, 0.74) | 0.03 (−1.00, 1.05) | 0.12 (−0.29, 0.53) | 0.21 (−0.20, 0.61) |
| Overweight | −0.32 (−0.86, 0.21) | −0.23 (−0.76, 0.30) | −0.253 (−0.46, −0.04) | −0.20 (−0.41, 0.009) |
| Obese | −1.613 (−2.40, −0.83) | −1.343 (−2.12, −0.56) | −0.693 (−1.00, −0.38) | −0.593 (−0.90, −0.28) |
| Rhea (n = 1103) | ||||
| Underweight | −1.04 (−2.17, 0.08) | −0.90 (−1.98, 0.19) | −0.35 (−0.70, 0.01) | −0.27 (−0.61, 0.08) |
| Overweight | −1.233 (−1.85, −0.61) | −1.283 (−1.87, −0.68) | −0.253 (−0.45, −0.06) | −0.293 (−0.48, −0.10) |
| Obese | −0.843 (−1.61, −0.07) | −0.56 (−1.30, 0.19) | −0.363 (−0.60, −0.11) | −0.293 (−0.53, −0.05) |
| Viva (n = 1705) | ||||
| Underweight | −2.013 (−3.99, −0.18) | −1.68 (−3.55, 0.19) | −0.773 (−1.32, −0.23) | −0.673 (−1.21 −0.14) |
| Overweight | −1.293 (−2.12, −0.47) | −1.103 (−1.91, −0.29) | −0.423 (−0.66, −0.19) | −0.333 (−0.56, −0.10) |
| Obes | −2.883 (−3.83, −1.93) | −2.523 (−3.46, −1.57) | −1.063 (−1.33, −0.78) | −0.873 (−1.14, −0.60) |
Underweight: <18.5 kg/m2, normal: 18.5 to <25 kg/m2, overweight 25 to <30 kg/m2, obese 30+ kg/m2. Analysis performed with linear regression, unadjusted and adjusted for confounders.
Abbreviation: INMA, INfancia y Medio Ambiente.
3P < 0.05.
Unadjusted estimates for the association between maternal BMI (categorical) and breastfeeding duration in months.
Adjusted estimates for maternal age, maternal race, maternal education, parity, and smoking during pregnancy.
Mediating factors in the association between maternal BMI and breastfeeding duration
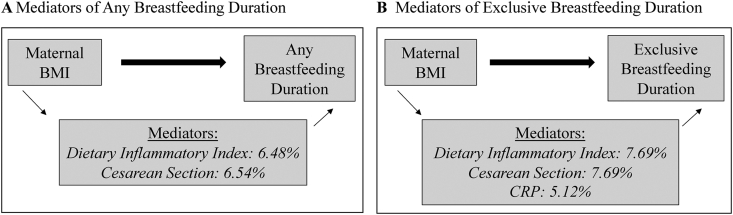
We found evidence for mediation in the association between maternal BMI and breastfeeding duration for DII, CRP, and cesarean delivery (FIGURE 2, FIGURE 3, Supplemental Figure 3, Supplemental Table 5). DII was found to mediate 6.48% of the relationship between maternal BMI and any breastfeeding duration (indirect effect: −0.07, 95% CI: −0.11, −0.04; total effect: −1.08, 95% CI: −1.35, −0.79) and cesarean delivery 6.54% (indirect effect: −0.07, 95% CI: −0.12, −0.04; total effect: −1.07, 95% CI: −1.35, −0.77). For exclusive breastfeeding duration, DII mediated 7.69% (indirect effect: −0.03, 95% CI: −0.04, −0.01; total effect: −0.39, 95% CI: −0.48, −0.30), cesarean delivery 7.69% (indirect effect: −0.03, 95% CI: −0.04, −0.02; total effect: −0.39, 95% CI: −0.48, −0.25), and CRP 5.12% (indirect effect: −0.02, 95% CI: −0.04, −0.002; total effect: −0.39, 95% CI: −0.50, −0.28).
FIGURE 2.
Forest plots of the indirect effect estimates for the mediating factors in the association between maternal BMI and breastfeeding duration. Mediation analysis with medeff. Gray dots represent beta estimate and black bars represent 95% CI. n = 5120. (A) Indirect effects of mediators in the association between maternal BMI and any breastfeeding duration. (B) Indirect effects of mediators in the association between maternal BMI and exclusive-breastfeeding duration. AGA, appropriate for gestational age; DII, dietary inflammatory index; GDM, gestational diabetes mellitus; LGA, large for gestational age; SGA, small for gestational age.
FIGURE 3.
Directed acyclic graph demonstrating percent mediation of the significant mediators in the association between maternal BMI category and breastfeeding duration. Mediation analysis with medeff. Bold arrows represent direct effects and thin arrows represent indirect effects. n = 5120. (A) Significant mediators in the association between maternal BMI and any breastfeeding duration. (B) Significant mediators in the association between maternal BMI and exclusive-breastfeeding duration.
Although CRP was a significant mediator in the relationship between maternal BMI and exclusive breastfeeding duration, the mediating association for CRP between maternal BMI and any breastfeeding duration did not reach statistical significance (indirect effect: −0.05, 95% CI: −0.11, −0.01; total effect: −1.08, 95% CI: −1.39, −0.74). Gestational age, GDM, and BW were also not found to be significant mediators in the association between maternal BMI and breastfeeding duration.
Because of the lack of information on breastfeeding intention in mothers who never breastfed, we performed subgroup analyses excluding subjects who never breastfed (n = 753) for all mediators that were significant in the entire cohort. The mediating effect of CRP in the associations between maternal BMI and exclusive breastfeeding duration was attenuated to the null. All other mediators remained significant, including DII and c-section, in the associations between maternal BMI and any and exclusive breastfeeding duration.
Sensitivity analyses
There was no significant interaction of infant gender or maternal marital status on the association between maternal BMI and breastfeeding outcome. Maternal education was found to have a significant interaction for any breastfeeding (interaction P = 0.003) and exclusive breastfeeding (interaction P = 0.04). The associations between maternal BMI and both breastfeeding outcomes were stronger among more highly educated women as compared with women with less than college education [any: −0.16 mo per 1 kg/m2 increase in BMI (95% CI: −0.22, −0.11) compared with. −0.06 mo per 1 kg/m2 increase in BMI (95% CI: −0.10, −0.02)].
Discussion
We pooled data from 3 geographically and culturally diverse mother–infant cohorts to investigate the association between maternal BMI and breastfeeding duration and identify mediators. Our findings robustly confirm the relationship between maternal BMI and breastfeeding duration and expand upon prior studies by finding that this association holds across global and culturally different cohorts. In addition, we found evidence that maternal inflammation, both dietary and systemic, and cesarean delivery partially mediate this association. These percentages of mediation are clinically important because identifying even small pieces of the causal pathways allows for public health interventions and funding to focus on key underlying mechanisms.
Prior literature has demonstrated an association between maternal obesity and breastfeeding duration. In a systematic review published in 2014, Turcksin et al. examined 19 single-nation studies from 7 different countries evaluating the relationship between maternal BMI and breastfeeding outcomes [51]. Eighteen of the studies investigated the role of maternal obesity on breastfeeding duration and 15 demonstrated shorter breastfeeding duration among women with obesity, 11 of which were statistically significant after adjusting for confounders. Women with obesity were also found to have a higher risk of early cessation of breastfeeding (hazard ratios 1.24–2.54) [51].
Although there are robust data regarding the association between maternal obesity and breastfeeding duration, data are limited on potential mechanisms and mediating factors underlying this association. Animal models have shown that obesity leads to failure of lactogenesis II across species [52,53], as well as changes in mammary gland development and anatomy [12,54]. Kelleher et al. showed that diet-induced obesity in mice led to the suppression of the mammary gland secretory function, in that 77% of obese mice failed to nurse their offspring past 5 d of lactation compared with 39% of normal-weight mice (<0.01) [12]. In addition, in the mammary gland of the obese mice, there were significantly higher levels of macrophages and proinflammatory markers compared with mammary glands of lean mice [12]. Importantly, they demonstrated increased mammary gland involution and autophagy of mammary epithelial cells in obese mice.
In our analysis, we showed that elevated maternal inflammation in humans, as measured by higher DII and CRP during pregnancy, is a mediator in the association of maternal prepregnancy BMI and breastfeeding outcomes. This finding supports what has been shown in animal models by Kelleher et al. [12] and in a prior analysis of the Project Viva cohort, where higher maternal inflammation was associated with shorter duration of breastfeeding [16]. Our study reinforced these findings across a larger and more diverse population with different dietary habits.
We also found cesarean delivery to be a significant mediator between maternal BMI and breastfeeding duration. Obesity is a known risk factor for necessitating cesarean delivery, and cesarean delivery has been shown to lead to a higher risk of breastfeeding failure [21,55]. Wu et al. have previously shown that women who underwent cesarean delivery had a higher risk of failing to continue any breastfeeding at 3 mo, with odds ratio 1.715 (95% CI: 1.265, 2.325) compared with women who delivered vaginally [55]. Interventions aimed at decreasing rates of cesarean deliveries among women with obesity could improve breastfeeding duration in this population.
Disruption in insulin and glucose metabolism has also been proposed as a mediator of delayed lactogenesis in women with obesity [56]. It has been shown that maternal GDM is associated with decreased breastfeeding duration even when controlling for maternal BMI, but data are still limited on the effects of insulin resistance and glucose intolerance on the mammary gland itself [57]. GDM is also associated with increased inflammation, which our study suggests may be the underlying mediator of this association. Interestingly, our study did not find GDM to be a significant mediator between maternal BMI and breastfeeding outcomes. However, our pooled cohort may have been underpowered given the relatively small number of women diagnosed with GDM in the study population.
Strengths and limitations
The strengths of this study include the multicultural context that is relevant to the exposure, outcome, and some mediators that are culturally pattered. Examining the association between maternal BMI and breastfeeding duration across multicultural cohorts provides generalizability of our findings and stronger evidence for potentially causal associations. Since breastfeeding rates are influenced by social and cultural factors, assessing this relationship in a diverse cohort is important. In addition, given the pooled sample size, we were able to leverage causal mediation techniques. Our data provide preliminary targets (dietary and systemic inflammation, and mode of delivery) for future clinical trials to improve breastfeeding duration in high-risk pregnant women with overweight and obesity.
Limitations include the lack of consistent variables collected across the 3 cohorts, particularly regarding factors that may influence breastfeeding, such as intention to breastfeed, length of maternity leave, and socioeconomic status. CRP data had a large percentage of missing values, in part because CRP was not measured in 2 INMA subcohorts, and therefore, our analysis evaluating the potential mediating role of inflammation had lower statistical power. DII was calculated at different times during gestation in each cohort. Although we have found that DII is highly correlated at different timepoints across pregnancy and we have adjusted for cohort, this remains a limitation of the available data. In our study, we have examined DII in pregnancy as a mediator between prepregnancy obesity and breastfeeding duration. However, the relationship between BMI and DII can be bidirectional, as DII before pregnancy may also influence subsequent BMI. We did not dispose DII measurements before pregnancy in this study. Future studies with repeated measurements of BMI and DII from preconception to pregnancy can be informative to clarify the DII role as either a mediator or a confounder in the prepregnancy BMI association with breastfeeding. Maternal weight was self-reported; however, literature has shown a strong correlation between self-reported and measured weight in early pregnancy [58,59]. We were limited in the available biomarkers that we could include as potential mediators, such as insulin and prolactin. Although we had a culturally diverse group of participants, our combined cohort lacked racial diversity. Our study population had lower rates of prepregnancy obesity and higher rates of education than general United States populations of pregnant woman, potentially limiting generalizability to populations with higher rates of obesity, although the biologic underpinnings and mechanisms remain relevant and similar. We were also underpowered in specific subgroups, such as women with GDM. Finally, because of the limitations of mediation analysis, which provides estimates for the total, direct, and indirect effects (product of the independent variable to the mediator and the mediator to the dependent variable), we did not estimate the individual associations between the independent variable and the mediators.
Conclusion
Breastfeeding is important in optimizing health outcomes for mothers and their infants [60,61], and here we report that mothers with overweight and obesity breastfeed for shorter duration than normal-weight women. We found that maternal factors, including dietary and systemic inflammation, and mode of delivery, mediate this association. Because increasing numbers of women entering pregnancy have overweight/obesity, this finding has important health implications for future generations. We acknowledge the challenges in changing the diets of women with obesity during the vulnerable prenatal period. However, data suggest that pregnant women may be more willing to change their diets during pregnancy than at other times to improve the health of their fetus [62], making pregnancy in some ways an ideal opportunity to initiate lifestyle modification. In addition, we acknowledge that there are far-reaching reasons beyond breastfeeding to limit cesarean sections, including maternal and infant morbidities associated with cesarean deliveries, and the data presented here provide further incremental support to minimize cesarean sections. These data additionally support the need for obstetricians, nutritionists, and lactation support to provide integrated prenatal counseling to address these interrelated domains for women who enter pregnancy with overweight or obesity. Taking this evidence into account, future studies should develop strategies to help women decrease dietary inflammation, encourage vaginal deliveries when possible, and achieve healthy prepregnancy weight in subsequent pregnancies, as ways to improve breastfeeding outcomes.
Acknowledgments
We most importantly thank the mothers and babies who participated in the 3 cohorts. Thank you also to all of the researchers involved in recruitment and data collection of the cohorts.
The authors report no conflicts of interest.
The authors’ responsibilities were as follows – MK, SS, DV, EO: conceptualized and designed the study; MK: performed the statistical analysis and drafted the manuscript; CA: contributed to the study design and statistical analysis and reviewed the manuscript; VM: provided statistical expertise, verified results, generated visuals, and reviewed the manuscript; DV, SS: supervised the study design and statistical analysis and reviewed the manuscript; PC, MG, AJ-R, MM, CR-D, DR, LS-M, MV, LC, EO: participated in recruitment and data collection for the cohorts and reviewed the manuscript; And all authors: read and approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.04.004.
Funding
This study was supported by T32 grant HD 098061 (MK) and P30ES023515 (DV, VM). The Project Viva cohort was funded by R01 HD 034568 (EO) and UH3 OD 023286 (EO). The INfancia y Medio Ambiente (INMA) cohort was funded by Grants from Instituto de Salud Carlos III and Spanish Ministry of Health (Red INMA G03/176; CB06/02/0041; FIS 97/0588; 00/0021-2, PI061756; PS0901958; FIS-FEDER 03/1615, 04/1509, 04/1112, 04/1931, 05/1079, 05/1052, 06/1213, 07/0314 and 09/02647; FIS-PI041436, FIS-PI081151, FIS-PI06/0867, FIS-PS09/00090, and CPII18/00018), Conselleria de Sanitat Generalitat Valenciana, Generalitat de Catalunya-CIRIT 1999SGR 00241, Department of Health of the Basque Government (2005111093 and 2009111069), the Provincial Government of Gipuzkoa (DFG06/004 and DFG08/001), and Fundación Roger Torné. ISGlobal acknowledges support from the Spanish Ministry of Science and Innovation and the State Research Agency through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. The “Rhea” project was financially supported by European projects (EU FP6-2003-Food-3-NewGeneris, EU FP6. STREP Hiwate, EU FP7 ENV.2007.1.2.2.2. Project No 211250 Escape, EU FP7-2008-ENV 1.2.1.4 Envirogenomarkers, EU FP7-HEALTH-2009-single-stage CHICOS, EU FP7 ENV.2008.1.2.1.6. Proposal No. 226285 ENRIECO, EU-FP7-HEALTH-2012 Proposal No. 308333 HELIX) and the Greek Ministry of Health (Program of Prevention of Obesity and Neurodevelopmental Disorders in Preschool Children, in Heraklion District, Crete, Greece: 2011–2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–2015). The Rhea study was also funded by Karolinska Institutet and the Swedish Research Council (Project No. 2015-03655). Additional funding from the National Institute of Environmental Health Science (NIEHS) supported LD (R01-ES029944, R01-ES030691, R01-ES030364, R21-ES028903, R21-ES029681, and P30-ES007048).
The funding sources of the initial cohorts and this analysis had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request, subject to approval from and regulations of each cohort.
Author disclosures
The authors report no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 2.Dieterich C.M., Felice J.P., O’Sullivan E., Rasmussen K.M. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr. Clin. North Am. 2013;60:31–48. doi: 10.1016/j.pcl.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC . 2020. Breastfeeding Report Card.https://www.cdc.gov/breastfeeding/data/reportcard.htm Internet. [Date last updated: August 31, 2022. Date cited: March 1, 2022]. Available from: [Google Scholar]
- 4.Theurich M.A., Davanzo R., Busck-Rasmussen M., Díaz-Gómez N.M., Brennan C., Kylberg E., et al. Breastfeeding rates and programs in Europe: a survey of 11 national breastfeeding committees and representatives. J. Pediatr. Gastroenterol. Nutr. 2019;68:400–407. doi: 10.1097/MPG.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 5.Flegal K.M., Kruszon-Moran D., Carroll M.D., Fryar C.D., Ogden C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driscoll A.K., Gregory E.C.W. 2020. Increases in prepregnancy obesity: United States, 2016–2019, NCHS Data Brief; pp. 1–8. [PubMed] [Google Scholar]
- 7.Rutishauser I.H., Carlin J.B. Body mass index and duration of breast feeding: a survival analysis during the first six months of life. J. Epidemiol. Community Health. 1992;46:559. doi: 10.1136/jech.46.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donath S., Amir L. Does maternal obesity adversely affect breastfeeding initiation and duration? J. Paediatr. Child Health. 2000;36:482–486. doi: 10.1046/j.1440-1754.2000.00562.x. [DOI] [PubMed] [Google Scholar]
- 9.Hilson J.A., Rasmussen K.M., Kjolhede C.L. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. J. Hum. Lact. 2004;20:18–29. doi: 10.1177/0890334403261345. [DOI] [PubMed] [Google Scholar]
- 10.Li R., Jewell S., Grummer-Strawn L. Maternal obesity and breast-feeding practices. Am. J. Clin. Nutr. 2003;77:931–936. doi: 10.1093/ajcn/77.4.931. [DOI] [PubMed] [Google Scholar]
- 11.Villar M., Santa-Marina L., Murcia M., Amiano P., Gimeno S., Ballester F., et al. Social factors associated with non-initiation and cessation of predominant breastfeeding in a mother–child cohort in Spain. Matern. Child Health J. 2018;22:725–734. doi: 10.1007/s10995-018-2441-1. [DOI] [PubMed] [Google Scholar]
- 12.Hennigar S.R., Velasquez V., Kelleher S.L. Obesity-induced inflammation is associated with alterations in subcellular zinc pools and premature mammary gland involution in lactating mice. J. Nutr. 2015;145:1999–2005. doi: 10.3945/jn.115.214122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woori N., Misung K., Cheongmin S. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in Korean: data from the health examinee cohort. J. Clin. Biochem. Nutr. 2018;62:17–22. doi: 10.3164/jcbn.17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 15.Aronson D., Bartha P., Zinder O., Kerner A., Markiewicz W., Avizohar O., et al. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int. J. Obesity Relat. Metab. Disord. 2004;28:674–679. doi: 10.1038/sj.ijo.0802609. [DOI] [PubMed] [Google Scholar]
- 16.Sen S., Rifas-Shiman S.L., Shivappa N., Wirth W.D., Hébert J.R., Gold D.R., et al. Dietary inflammatory potential during pregnancy is associated with lower fetal growth and breastfeeding failure: results from Project Viva. J. Nutr. 2015;146:728–736. doi: 10.3945/jn.115.225581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen P.T.H., Binns C.W., Nguyen C.L., Ha A.V.V., Chu T.K., Duong DV D.V., et al. Gestational diabetes mellitus reduces breastfeeding duration: a prospective cohort study. Breastfeed Med. 2019;14:39–45. doi: 10.1089/bfm.2018.0112. [DOI] [PubMed] [Google Scholar]
- 18.Bryanton J., Montelpare W., Drake P., Drake R., Walsh D., Larter K. Relationships among factors related to childbirth and breastfeeding outcomes in primiparous women. J. Obstet. Gynecol. Neonatal Nurs. 2020;49:437–451. doi: 10.1016/j.jogn.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Cordero L., Oza-Frank R., Landon M.B., Nankervis C.A. Breastfeeding initiation among macrosomic infants born to obese nondiabetic mothers. Breastfeed Med. 2015;10:239–245. doi: 10.1089/bfm.2015.0028. [DOI] [PubMed] [Google Scholar]
- 20.Leonard S.A., Rasmussen K.M. Larger infant size at birth reduces the negative association between maternal prepregnancy body mass index and breastfeeding duration. J. Nutr. 2011;141:645–653. doi: 10.3945/jn.110.129874. [DOI] [PubMed] [Google Scholar]
- 21.Hobbs A.J., Mannion C.A., McDonald S.W., Brockway M., Tough S.C. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth. 2016;16:90. doi: 10.1186/s12884-016-0876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guxens M., Ballester F., Espada M., Fernández M.F., Grimalt J.O., Ibarluzea J., et al. Cohort profile: the INMA—INfancia y Medio Ambiente—(Environment and Childhood) Project. Int. J. Epidemiol. 2012;41:930–940. doi: 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- 23.Chatzi L., Leventakou V., Vafeiadi M., Koutra K., Roumeliotaki T., Chalkiadaki G., et al. Cohort profile: the mother-child cohort in Crete, Greece (Rhea Study) Int. J. Epidemiol. 2017;46:1392–1393. doi: 10.1093/ije/dyx084. [DOI] [PubMed] [Google Scholar]
- 24.Oken E., Baccarelli A.A., Gold D.R., Kleinman K.P., Litonjua A.A., Meo D.D., et al. Cohort profile: project viva. Int. J. Epidemiol. 2014;44:37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir C.B., Jan A. StatPearls Publishing; Treasure Island (FL): 2021. BMI classification percentile and cut off points. StatPearls [Internet] [PubMed] [Google Scholar]
- 26.Boucher O., Julvez J., Guxens M., Arranz E., Ibarluzea J., de Miguel M.S., et al. Association between breastfeeding duration and cognitive development, autistic traits and ADHD symptoms: a multicenter study in Spain. Pediatr. Res. 2017;81:434–442. doi: 10.1038/pr.2016.238. [DOI] [PubMed] [Google Scholar]
- 27.Vassilaki M., Chatzi L., Bagkeris E., Papadopoulou E., Karachaliou M., Koutis A., et al. Smoking and caesarean deliveries: major negative predictors for breastfeeding in the mother–child cohort in Crete, Greece (Rhea study), Matern. Child Nutr. 2014;10:335–346. doi: 10.1111/j.1740-8709.2012.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belfort M.B., Rifas-Shiman S.L., Kleinman K.P., Guthrie L.B., Bellinger D.C., Taveras E.M., et al. Infant feeding and childhood cognition at ages 3 and 7 years: effects of breastfeeding duration and exclusivity. JAMA Pediatr. 2013;167:836–844. doi: 10.1001/jamapediatrics.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization . Division of Child Health and Development; 1991. Indicators for Assessing Breastfeeding Practices.https://www.unscn.org/web/archives_resources/files/Indicators_for_assessing_breastfeed_791.pdf [Internet] Available from: [Google Scholar]
- 30.Greenland S., Pearl J., Robins J.M. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 31.Spinelli S., Monteleone E. Food preferences and obesity. Endocrinol. Metab. (Seoul). 2021;36:209–219. doi: 10.3803/EnM.2021.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatzi L., Plana E., Pappas A., Alegkakis D., Karakosta P., Daraki V., et al. The metabolic syndrome in early pregnancy and risk of gestational diabetes mellitus. Diabetes Metab. 2009;35:490–494. doi: 10.1016/j.diabet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Catalano P.M., Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ Clin. Res. Ed. 2017;356:j1. doi: 10.1136/bmj.j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cnattingius S., Villamor E., Johansson S., Bonamy A.-K.E., Persson M., Wikström A.-K., et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309:2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 35.Keenan-Devlin L.S., Awosemusi Y.F., Grobman W., Simhan H., Adam E., Culhane J., et al. Early term delivery and breastfeeding outcomes. Matern. Child Health J. 2019;23:1339–1347. doi: 10.1007/s10995-019-02787-4. [DOI] [PubMed] [Google Scholar]
- 36.Nelson S.M., Matthews P., Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum. Reprod. Update. 2009;16:255–275. doi: 10.1093/humupd/dmp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y.B., Shivappa N., Hébert J.R., Page A.J., Gill T.K., Melaku Y.A. Association between dietary inflammatory index, dietary patterns, plant-based dietary index and the risk of obesity. Nutrients. 2021;13:1536. doi: 10.3390/nu13051536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matilla-Santander N., Valvi D., Lopez-Espinosa M.-J., Manzano-Salgado C.B., Ballester F., Ibarluzea J., et al. Exposure to perfluoroalkyl substances and metabolic outcomes in pregnant women: evidence from the Spanish INMA Birth Cohorts. Environ. Health Perspect. 2017;125:117004. doi: 10.1289/EHP1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daraki V., Georgiou V., Papavasiliou S., Chalkiadaki G., Karahaliou M., Koinaki S., et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: the Rhea Pregnancy Cohort Crete, Greece. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0126327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivappa N., Steck S.E., Hurley T.G., Hussey J.R., Hébert J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández-Barrés S., Romaguera D., Valvi D., Martínez D., Vioque J., Navarrete-Muñoz E.M., et al. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: the INMA birth cohort study. Pediatr. Obes. 2016;11:491–499. doi: 10.1111/ijpo.12092. [DOI] [PubMed] [Google Scholar]
- 42.Lertxundi N., Molinuevo A., Valvi D., Gorostiaga A., Balluerka N., Shivappa N., et al. Dietary inflammatory index of mothers during pregnancy and Attention Deficit-Hyperactivity Disorder symptoms in the child at preschool age: a prospective investigation in the INMA and RHEA cohorts. Eur. Child Adolesc. Psychiatry. 2022;31:615–624. doi: 10.1007/s00787-020-01705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rammah A., Whitworth K.W., Amos C.I., Estarlich M., Guxens M., Ibarluzea J., et al. Air pollution, residential greenness and metabolic dysfunction during early pregnancy in the INfancia y Medio Ambiente (INMA) Cohort. Int. J. Environ. Res. Public Health. 2021;18:9354. doi: 10.3390/ijerph18179354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleisch A.F., Gold D.R., Rifas-Shiman S.L., Koutrakis P., Schwartz J.D., Kloog I., et al. Air pollution exposure and abnormal glucose tolerance during pregnancy: the Project Viva Cohort. Environ. Health Perspect. 2014;122:378–383. doi: 10.1289/ehp.1307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 46.Papageorghiou A.T., Kennedy S.H., Salomon L.J., Altman D.G., Ohuma E.O., Stones W., et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am. J. Obstet. Gynecol. 2018;218:S630–S640. doi: 10.1016/j.ajog.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Hicks R., Tingley D. Causal mediation analysis. STATA J. 2011;11:605–619. [Google Scholar]
- 48.VanderWeele T.J. On the relative nature of overadjustment and unnecessary adjustment. Epidemiology. 2009;20:496–499. doi: 10.1097/EDE.0b013e3181a82f12. [DOI] [PubMed] [Google Scholar]
- 49.Whitcomb B.W., Schisterman E.F., Perkins N.J., Platt R.W. Quantification of collider-stratification bias and the birthweight paradox. Paediatr. Perinat. Epidemiol. 2009;23:394–402. doi: 10.1111/j.1365-3016.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Multiple Imputation in Stata https://stats.idre.ucla.edu/stata/seminars/mi_in_stata_pt1_new/ [Internet]. [Date last updated: 2021. Date cited: April 30, 2021]. Available from:
- 51.Turcksin R., Bel S., Galjaard S., Devlieger R. Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern. Child Nutr. 2014;10:166–183. doi: 10.1111/j.1740-8709.2012.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace J.M., Milne J.S., Aitken R.P. The effect of overnourishing singleton-bearing adult ewes on nutrient partitioning to the gravid uterus. Br. J. Nutr. 2005;94:533–539. doi: 10.1079/bjn20041398. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen K.M. Association of maternal obesity before conception with poor lactation performance. Annu. Rev. Nutr. 2007;27:103–121. doi: 10.1146/annurev.nutr.27.061406.093738. [DOI] [PubMed] [Google Scholar]
- 54.Flint D.J., Travers M.T., Barber M.C., Binart N., Kelly P.A. Diet-induced obesity impairs mammary development and lactogenesis in murine mammary gland. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1179–E1187. doi: 10.1152/ajpendo.00433.2004. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y., Wang Y., Huang J., Zhang Z., Wang J., Zhou L., et al. The association between caesarean delivery and the initiation and duration of breastfeeding: a prospective cohort study in China. Eur. J. Clin. Nutr. 2018;72:1644–1654. doi: 10.1038/s41430-018-0127-9. [DOI] [PubMed] [Google Scholar]
- 56.Nommsen-Rivers L.A., Dolan L.M., Huang B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med. 2012;7:43–49. doi: 10.1089/bfm.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nommsen-Rivers L.A. Does insulin explain the relation between maternal obesity and poor lactation outcomes? An overview of the literature. Adv. Nutr. 2016;7:407–414. doi: 10.3945/an.115.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carrilho T.R.B., Rasmussen K.M., Farias D.R., Costa N.C.F., Batalha M.A., Reichenheim M.E., et al. Agreement between self-reported pre-pregnancy weight and measured first-trimester weight in Brazilian women. BMC Pregnancy Childbirth. 2020;20:734. doi: 10.1186/s12884-020-03354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Provenzano A.M., Rifas-Shiman S.L., Herring S.J., Rich-Edwards J.W., Oken E. Associations of maternal material hardships during childhood and adulthood with prepregnancy weight, gestational weight gain, and postpartum weight retention. J. Womens Health (Larchmt). 2015;24:563–571. doi: 10.1089/jwh.2014.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramer M.S., Chalmers B., Hodnett E.D., Sevkovskaya Z., Dzikovich I., Shapiro S., et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 61.Duijts L., Jaddoe V.W.V., Hofman A., Moll H.A. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18–e25. doi: 10.1542/peds.2008-3256. [DOI] [PubMed] [Google Scholar]
- 62.Forbes L.E., Graham J.E., Berglund C., Bell R.C. Dietary change during pregnancy and women’s reasons for change. Nutrients. 2018;10:1032. doi: 10.3390/nu10081032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request, subject to approval from and regulations of each cohort.