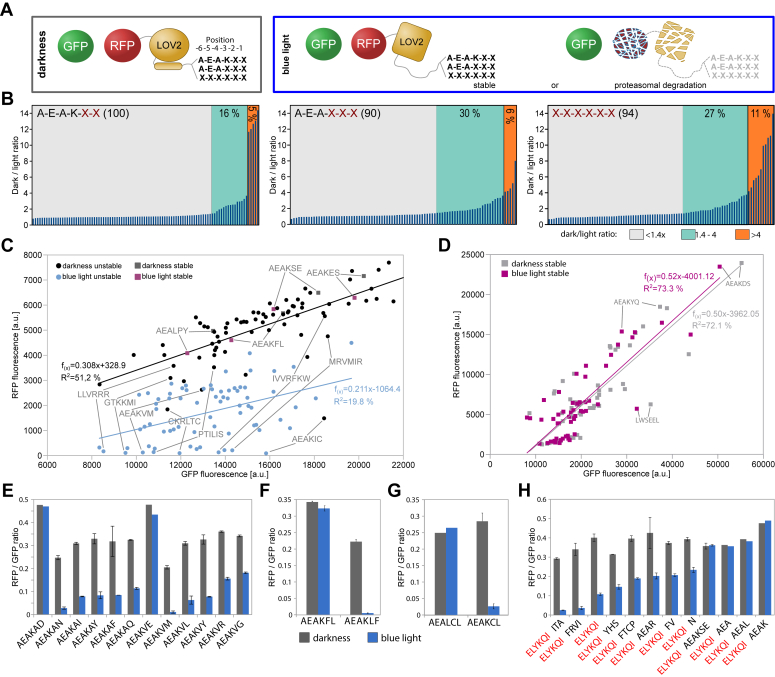
Figure 1.
Influence of light-exposed short random C-terminal peptides on protein stability measured with a split tandem fluorescent protein reporter.A, schematic of the LOV2-based system to characterize the influence of different C termini on the stability of the fluorescence reporter mScarlettI (RFP). Blue light–induced unfolding of the Jɑ-helix of the LOV2 domain unmasks the C terminus and might be recognized by protein quality control. The sfGFP was used as a protein biosynthesis reference. The last two, three, or six amino acids of the C terminus were randomly exchanged within the GFP-P2A-RFP-LOV2 construct to generate CtPC variants. B, distribution of switching ratios between light conditions of randomly picked CtPC variants. Cells carrying the construct sfGFP-P2A-RFP-LOV2 with randomly generated C termini were randomly selected. The RFP–GFP ratio of the selected clones was determined after 5 h in darkness and blue light. The dark–light switching ratios are shown for randomly selected clones with exchanges of two, three, and six amino acids. Variants were ordered by their switching ratios. Numbers in brackets indicate the number of tested clones. C and D, plot of fluorescence intensity of GFP and RFP of CtPC-degrons (C) and stable CtPC variants (D). Wildtype (ESM356-1) cells containing CtPC plasmids were incubated in darkness or blue light for 5 h prior to the measurements. Selected variants are highlighted. Linear fits for both conditions are shown together with the value for R-squared that indicates how well the linear regression model fits the data. E–H, selected examples of CtPC variants. RFP–GFP ratios are shown in the graphs. The error bars show the standard deviation (n = 3). CtPC, C-terminal peptide collection; LOV2, light–oxygen–voltage 2; RFP, red fluorescent protein; SfGFP, superfolder GFP.