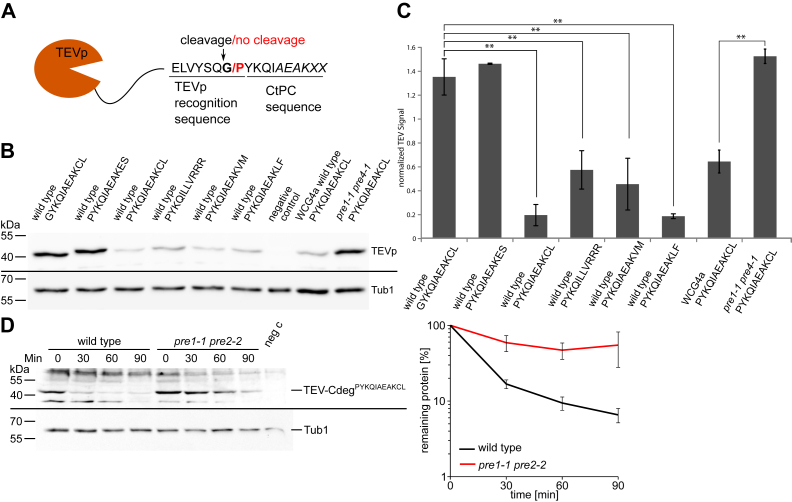
Figure 2.
CtPC-degrons are transferable.A, fusion of the last 10 amino acids of different CtPC variants to the C terminus of the TEV protease. The linker contains a TEV cleavage site (ELVYSQ-G) or a mutated sequence (ELVYSQ-P). Depending on the composition of the recognition motif, the last 10 amino acids are cleaved away (sequence with G) or not (sequence with P). B, immunodetection of TEV-ELVYSQ-G/P-CtPC fusion proteins in wildtype strains ESM356-1, WCG4a, and in the strain WCG4a/11/22 (pre1-1 pre2-2). C, quantification of the respective TEVp signal detected by αTEV antibody. Significance was determined via Student’s t tests (∗∗p < 0.01). The error bars indicate the SEM (n = 3). D, the translational inhibitor cycloheximide was used to stop protein synthesis in a wildtype strain (WCG4a) and a proteasomal mutant strain (pre1-1 pre2-2). The degradation of TEVp-PYQIAEAKCL was followed in both strains by Western blotting. The graph shows the quantification of four independent measurements of TEVp abundance normalized to Tub1p levels (n = 4; error bars = SEM). CtPC, C-terminal peptide collection; TEVp, tobacco etch virus protease.