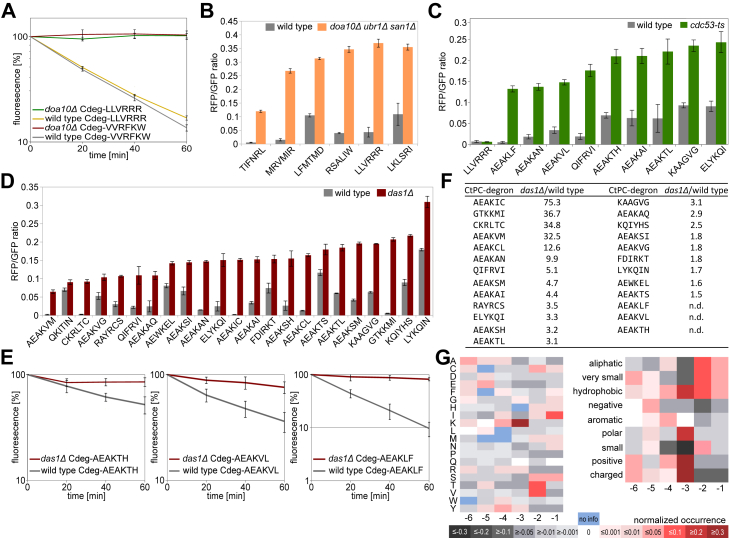
Figure 5.
Identification of ubiquitin–protein ligases involved in CtPC-degron degradation.A, cycloheximide chase of C-degrons in BY4741 (wildtype) and MHY1631 (doa10Δ) strain under blue light. Error bars show the standard deviation (n = 6). B, RFP/GFP fluorescence intensity ratios of RFP–LOV2–CtPC variants in BY4741 (wildtype) and YSH31 (doa10Δ san1Δ ubr1Δ) cells after 5 h blue light incubation. C, RFP/GFP ratios of RFP–LOV2–CtPC fusions in wildtype and TSA974 (Cdc53ts) cells. After 3 h of incubation, cells were shifted for 2 h to 37 °C. D, RFP/GFP ratios of RFP–LOV2–CtPC fusions in BY4741 (wildtype) and Y01276 (das1Δ). E, cycloheximide chases of CtPC-AEAKLF, CtPC-AEAKVL, and CtPC-AEAKTH variants in wildtype and Y01276 (das1Δ) cells. Error bars show the standard deviation (n = 6). F, overview of CtPC-degrons classified as mainly Das1 dependent based on the Y01276 (das1Δ) to wildtype ratio. A ratio greater than 1.5 was classified as mainly Das1-dependent degradation, one between 1.5 and 1.2 was considered a slight Das1-influence degradation, and a ratio less than 1.2 was classified as Das1-independent degradation (Table S3). G, appearance of amino acids at specific positions and amino acid properties in Das1-dependent C-degrons (das1Δ/wildtype: >1.5) normalized to Das1-independent C-degrons (das1Δ/wildtype: <1.2) and stable CtPC variants. Values above zero (shades of red) indicate increased occurrence in Das1-dependent C-degrons, and values below zero (shades of black) indicate increased occurrence in stable variants or Das1-independent degrons. CtPC, C-terminal peptide collection; LOV2, light–oxygen–voltage 2; n.d., not determined; RFP, red fluorescent protein.