FIGURE 5.
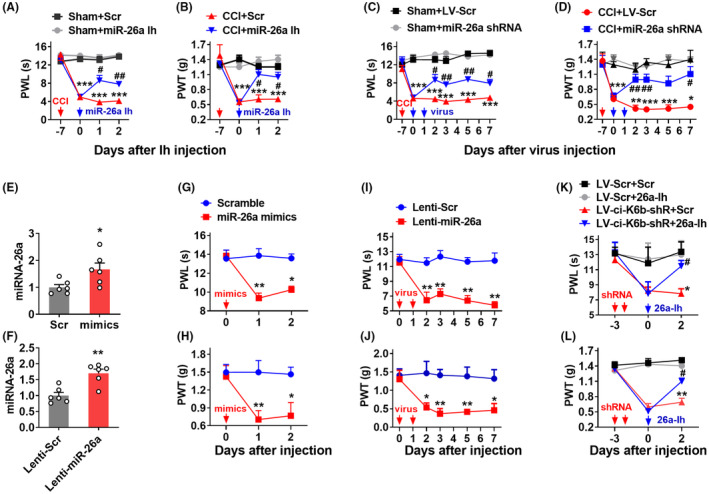
MiRNA‐26a upregulation contributes to neuropathic pain. (A, B) The intrathecal injections of miRNA‐26a inhibitor (miR‐26a Ih) but not its control scramble inhibitor (Scr) for 1 day alleviated the CCI‐induced thermal hyperalgesia (A) and mechanical allodynia (B). n = 8 mice/group. ***p < 0.001 versus the Sham plus Scr‐treated mice at the corresponding time points by two‐way ANOVA with repeated measures followed by post hoc Tukey test; # p < 0.05, ## p < 0.01 versus the CCI plus Scr‐treated mice at the corresponding time points by two‐way ANOVA with repeated measures followed by post hoc Tukey test. Red arrow indicates CCI or Sham surgery. Blue arrows indicate miR‐26a Ih or Scr. (C, D) 2 consecutive days intrathecal injections of Lenti‐miRNA‐26a inhibitor (miR‐26a shRNA) but not control lentivirus‐mediated scramble inhibitor (LV‐Scr) attenuated thermal hyperalgesia (C) and mechanical allodynia (D) in CCI mice. n = 8 mice/group. *p < 0.05, ***p < 0.001 versus Sham plus LV‐Scr‐treated mice at the corresponding time points by two‐way ANOVA with repeated measures followed by post hoc Tukey test; # p < 0.05, ## p < 0.01 versus CCI plus LV‐Scr‐treated mice at the corresponding time points by two‐way ANOVA with repeated measures followed by post hoc Tukey test. Red arrow indicates CCI or Sham surgery. Blue arrows indicate miR‐26a shRNA or LV‐Scr. (E) Levels of miRNA‐26a in the dorsal spinal cord on day 2 after intrathecal injection with miRNA‐26a mimics (mimics) or control scrambled miRNA (Scr) into spinal cord. n = 6 mice/group. *p < 0.05 versus the scrambled miRNA group by two‐tailed unpaired Student's t‐test. (F) Levels of miRNA‐26a in the dorsal spinal cord on day 5 after 2 consecutive days of intrathecal injection with Lenti‐miRNA‐26a (Lenti‐miR‐26a) or control scrambled virus (Lenti‐Scr) into the spinal cord. n = 6 mice/group. **p < 0.01 versus the scrambled virus group by two‐tailed unpaired Student's t‐test. (G, H) Intrathecal injection of miRNA‐26a mimics (miR‐26a mimics) or control scrambled miRNA (Scramble) into the spinal cord increased the hypersensitive response to heat stimuli (G) and to von Frey filaments stimuli (H) on the different days after mimics injection. n = 8 mice/group. *p < 0.05, **p < 0.01 versus the scrambled‐treated mice at the corresponding time points by two‐way ANOVA with repeated measures followed by post hoc Tukey test. The red arrow indicates mimics or Scr injection. (I, J) Two consecutive days of intrathecal injection of Lenti‐miRNA‐26a (Lenti‐miR‐26a) or control scrambled virus (Lenti‐Scr) into the spinal cord increased the hypersensitivity to heat stimuli (I) and to von Frey filaments stimuli (J) at the different days after lentivirus injection. n = 8 mice/group. *p < 0.05, **p < 0.01 versus the Lenti‐Scr‐treated mice at the corresponding time points by two‐way ANOVA with repeated measures followed by post hoc Tukey test. The red arrow indicates Lenti‐miR‐26a or Lenti‐Scr. (K, L) Two consecutive days intrathecal injection of miRNA‐26a inhibitor (26a‐Ih) on day 3 after intrathecal injection of Lenti‐ciRNA‐Kat6b‐shRNA (LV‐ci‐K6b‐shR) or its scrambled virus control (LV‐Scr) in naïve mice inhibited the production of pain hypersensitivity to heat stimuli (K) and to von Frey filaments stimuli (L). n = 8 mice/group. *p < 0.05, **p < 0.01 versus the LV‐Scr plus the scrambled inhibitor (Scr) group; # p < 0.05 versus the LV‐ci‐K6b‐shR plus Scr group by two‐way ANOVA with repeated measures followed by post hoc Tukey test. The red arrow indicates LV‐ci‐K6b‐shR or LV‐Scr injection. Blue arrows indicate 26a‐Ih or its control.
