Abstract
Background:
Inflammatory markers play a substantial role in the prognosis of breast cancer (BC). Studies have been conducted, evaluating the effect of yoga intervention (YI) on inflammatory biomarkers among BC cases. This systematic review consolidates the outcome of YI in the cancer microenvironment.
Objective:
The objective of the study was to evaluate the effect of YI in the cancer microenvironment among BC women.
Materials and Methods:
This review was conducted from May 2021 to December 2021. The inclusion criteria were experimental studies on adult BC cases with isolated YI. Studies conducted among paediatrics, case reports and case series were excluded from the study. Medline (PubMed), Medline (Ovid), Web of Science (WOS), Scopus, CINAHL and Cochrane Central databases were searched. The data were restricted from January 2000 to December 2021 with studies published in English. ‘The Cochrane risk of bias assessment tool’ was mobilised to evaluate the quality of the included studies.
Results:
A total of nine studies met the inclusion criteria and comprised a sample size of 905 BC cases with a mean age of 50.26±8.27 years. Three studies evaluated tumour necrosis factor-alpha (TNF-α) and INTERLEUKIN (IL)-6, where two studies on TNF-α and one on IL-6 favoured the YI group. A study investigated soluble TNF receptor II (TNF-RII) and another on IL-1beta (IL-1β) has shown improved levels post-YI. A downward trend of cortisol levels was noted in four out of five studies. Two studies that examined the C-reactive protein and a study on IL-8 did not show any difference between the YI and the control groups.
Conclusion:
This review’s findings showed the downregulation of cortisol, markers of inflammation; TNF-α, IL-6, TNF-RII and IL-1β immediately to post-YI. Heterogeneities in terms of YIs, number of days of practice, duration and training received and the grade of BC cases are the concern of this review. However, YI can be considered a supportive therapy for BC.
Keywords: Breast tumour, Tumour microenvironment, Inflammatory markers, Stress hormone, Yoga
INTRODUCTION
Breast cancer (BC) cases constitute 25.1% of all cancers in women.[1] In the year 2020, the World Health Organization reported more than 2 million cases and 6.85 lakh deaths.[2] The United States of America (USA) reported 281,550 new BC cases, with 43,600 deaths in 2021.[3] The incidence of BC cases in East Asian countries was 979,675 in 2019.[4] The National Cancer Registry Programme 2020 report estimated the BC in India to be 13.9 lakh and is likely to be increased to 15.7 lakhs by 2025.[5] In the past 25 years, a hike in mortality rate was reported globally, with a rise in the developing and low-income countries attributed to seeking last-hour medical care, a dearth of awareness on BC and an inability to access healthcare.[6,7]
Inflammatory responses are initiated by a coordinated effort of leucocytes and cytokines; when stress of any kind is induced, it affects the body in various ways by multiple biochemical mediated pathways. Inflammatory biomarkers such as interleukins (ILs), tumour necrosis factors and interferons originate from the proteins family and sketch a significant part of the advancement of BC. During the inflammatory process, the inflammatory markers play a protective role by upregulating the anti-inflammatory markers and downregulating the pro-inflammatory markers, and the other way around in the offensive role.
Cortisol, a glucocorticoid regulated by the hypothalamic-pituitary-adrenal axis, plays a significant part in maintaining homeostasis during stress; it protects the body in acute stress with its anti-inflammatory role but becomes detrimental to health in chronic stress with prolonged elevated cortisol. Clinical and molecular studies have reported a positive correlation between the increased level of cortisol and BC progression.
Stressed populations have abnormal levels of cortisol, TNF-α and IL-6;[8] these markers were found to be elevated in BC survivors when subjected to stress.[9] Depression is correlated to elevated levels of IL-1beta (IL-1β) and TNF-α among women with BC post-primary treatment.[10] IL-1β is a potential biomarker for increased risk of bone metastasis in BC.[11]
The interplay of genetic mutation and exposure to various exogenous and endogenous factors disrupts the endocrine functions and influences BC development.[12] Studies have estimated, 20% of BC is caused by genetic mutations such as BC genes 1 and 2 (BRCA 1 and BRCA 2), tumour protein 53, phosphatase-tensin homolog and ataxia-telangiectasia mutated.[13] Psychosocial causes, depression, stress and anti-emotions are contributing factors to BC development;[14] the progression of BC is correlated to stress and chronic depression.[15] Progression of cancer through sympathetic nervous system activation enhanced the production of inflammatory cytokines.[16,17]
A woman diagnosed with cancer of the breast is a stressful event irrespective of the stage. YI, meditation and relaxation therapies during BC treatment decrease the level of stress.[18] Mindfulness-based stress reduction programmes among survivors of BC found a dropped in cortisol levels (saliva) and IL-6 compared to the control group.[19] YI enhances psychological health during BC treatment.[20]
YI, a booming research interest with its origin in India, has found its way in effectively managing stress/anxiety, depression/mood disorders and improved quality of life/ physical functioning,[21,22] chemotherapy-induced nausea, vomiting,[23] lymphoedema, peripheral neuropathy, pain and sleep disturbances, caused by both emotional and physiological distress during and after cancer treatment.[18] Not much is known about its impact on the cancer microenvironment. This review reports the findings of YI in the tumour microenvironment.
MATERIAL AND METHODS
Basis of study selection
Inclusion criteria
Experimental studies (true experimental, quasi-experimental and pre-experimental) conducted among adult BC, outcomes addressed in terms of inflammatory biomarkers and cortisol was considered. Studies published between the year 2000 and 2021 in English language were included.
Exclusion criteria
Studies conducted among adult non-BC population, case reports and case series, interventions not inclusive of YI and where the outcome measures were not inflammatory markers or cortisol were excluded from this review.
The databases searched – were Medline (PubMed), Medline (Ovid), WOS, Scopus, CINAHL and Cochrane central. Studies published between the year 2000 and 2021 were included.
Search strategy
The databases searched were Medline (PubMed and Ovid), WOS, Scopus, CINAHL, and Cochrane central. MeSH terms and boolean operators were used as appropriate for different databases.
Population
‘Breast neoplasm*’ OR ‘Breast tumour*’ OR ‘Breast tumour*’ OR ‘breast carcinoma*’ OR ‘inflammatory breast neoplasm*’ OR ‘Breast cancer’ OR ‘breast malignant*’ OR ‘cancer of breast*’ OR ‘Inflammatory breast cancer.’
Intervention
‘Yoga’ OR ‘mind body therapy*’ OR ‘gentle yoga’ OR ‘Anuloma-Viloma’ OR ‘Anuloma Viloma’ OR ‘suryanamaskar’ OR ‘suryanamaskara’ OR ‘Suryanamaskar’ OR ‘asana*’ OR ‘pranayama*’ OR ‘Hatha Yoga’ OR ‘Raja Yoga’ OR ‘Iyengar Yoga’ OR ‘Shavasana’ OR ‘bhramari pranayama’ OR ‘nostril breathing*’ OR ‘Alternate nostril breathing*’ OR ‘kapalabhati’ OR ‘yoga therapy*’ OR ‘mind body therapy.*’
Outcomes
‘Biomarker*’ OR ‘biological marker*’ OR ‘inflammatory marker*’ OR ‘anti-inflammatory marker*’ OR ‘pro inflammatory marker*’ OR ‘interleukin*’ OR ‘cytokine*’ OR ‘chemokine*’ OR ‘Tumour necrosis factor’ OR ‘tumour necrosis factor alpha’ OR ‘tumour necrosis factor alpha’ OR ‘TNF alpha’ OR ‘TNF alpha’ OR ‘TNF alpha’ OR ‘annexin-a1’ OR ‘annexin-a2’ OR ‘IL10’ OR ‘IL1beta’ OR ‘IL4’ OR ‘IL6’ OR ‘IL8’ OR ‘IL-1beta’ OR ‘IL-4’ OR ‘IL-6’ OR ‘IL-8’ OR ‘IL-10’ OR ‘annexin-a1’ OR ‘annexin-a2’ OR ‘Interleukin-1beta’ OR ‘Interleukin-4’ OR ‘Interleukin-6’ OR ‘Interleukin-8’ OR ‘Interleukin-10’ OR ‘CRP’ OR ‘C-reactive protein’ OR ‘C-reactive protein’ OR ‘Cortisol’ OR ‘diurnal cortisol’ OR ‘Immunologic’ OR ‘inflammatory factor.*’
Data extraction
Data were extracted from the qualified studies into the preformatted datasheet by two reviewers independently. The characteristics of a study include (a) authors, publication year and country; (b) demographics of the study subjects as age and type and grade of BC; (c) study design and sample size; (d) intervention type, duration and intervention provider; (e) inflammatory markers/stress hormone and units of measurement and (f) study findings.
Risk of bias (RoB) assessment
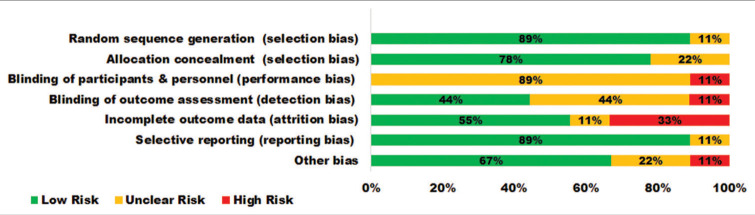
The quality of each qualified study was appraised using the Cochrane RoB 2 tool for randomised trials by Higgins et al.[24] It assesses the following aspects: Random sequence generation, allocation concealment, blinding of the outcome, incomplete data, selective reporting and other bias; using the grades as ‘Low risk’, ‘High risk’ and ‘Unclear risk’ indicating unknown risk of bias. The findings are projected in the RoB graph [Figure 1].
Figure 1:
Risk of bias graph.
Statistical analysis
For meta-analysis, mean difference with a 95% confidence interval was computed.[34] The Review Manager Software (RevMan 5, Cochrane Collaboration, Oxford, England) was used for data analytics.[35]
RESULTS
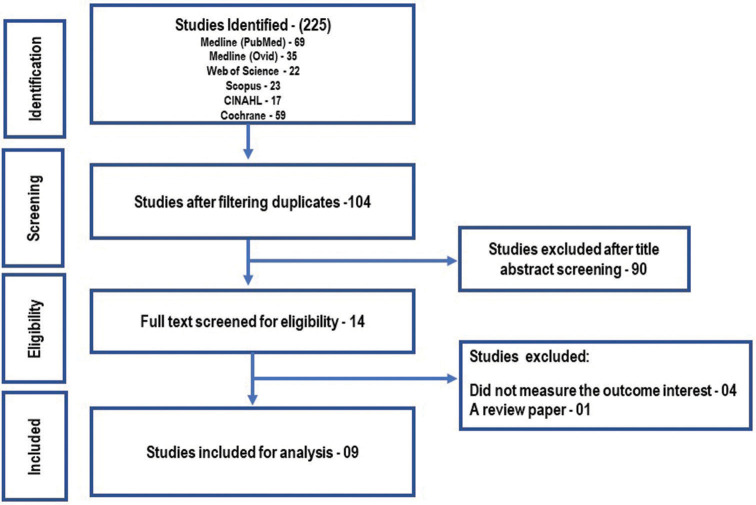
Overall, 225 citations were identified in the initial search, of which 121 were duplicates. Out of 104 records, 90 studies were excluded after screening titles and abstracts. Of the remaining 14 studies, nine articles were put through the review [Figure 2] which contributed to a sample size of 905 BC cases.[25-33] Five studies were conducted in the USA[27,29-32] and four in India.[25,26,28,33] Study subjects consist of BC survivors 38%, with stages ranging from 0 to IV;[27,29,31,32] undergoing radiotherapy 28%;[26,30] undergoing surgery 07%[25] and metastatic BC 7%;[28,33] the average age of the BC cases ranged from 46.8 to 63.33. All studies were randomised control trials (RCTs). Sample size (N) ranged from 18 to 200. The details of each study are summed up in Table 1.
Figure 2:
PRISMA flowchart.
Table 1:
Characteristics of the included studies.
| Authors | Year and country | Subject age (Mean; SD/SE) | Type and grade of BC | Study design and sample size | Yoga intervention duration and provider | Outcome with units of measurement | Study findings |
|---|---|---|---|---|---|---|---|
| Rao et al. | 2008; India | Study participants: 49.2±9.6 | Stages II and III BC undergoing surgery |
Two-arm RCT; (N) 69; (n=36): SC and Ex; (n=33): Integrated yoga | Half an hour daily for 4 weeks. Interventions were imparted by trained personnel. | TNF-α (pg/mL). IL-2r alpha; IFN-gamma |
Mean and SD of pre-and post-surgery TNF-αin: Control group: 18.5±52.1, 26±53.5; yoga group: 11.7±13.3, 8.5±7.2; (P<0.001) IL-2r alpha; IFN-gamma: reported as not significant between the group. |
| Vadiraja et al. |
2009; India | Supportive therapy: 48.45±10.21 Integrated yoga: 46±9.13 |
Stages II and III; undergoing adjuvant radiotherapy |
Two-arm RCT; (N) 88; (n=44): Supportive therapy; (n=44): Integrated yoga | 60 min of integrated yoga in person, thrice weekly for 6 wks. administered by a yoga therapist | Cortisol (saliva) (μg/dl) | Pooled mean (SD) cortisol in IY: 0.25 (0.13), 0.19 (0.13); S. Therapy: 0.25 (0.21), 0.25 (0.18); pre-and post-intervention, respectively. |
| Banasik et al. |
2011; USA | Control: 62.4±7.3 Experimental: 63.33±6.9 |
Stages II and IV BC survivors | Two-arm RCT; (N) 18, (n=9): Regular routines; (n=9): Iyengar yoga | 90 min Iyengar yoga, twice weekly for 8 weeks administered by Iyengar yoga instructor. | Cortisol (Salivary) pg/mL |
Significant decrease of cortisol in the morning (P=0.018) and 5 p.m. (P=0.028) in Iyengar yoga. |
| Neeta et al. |
2013; India | RT+C: 48.2±9.4 SK and P+C: 46.8±9.4 |
Stages IIb, III and IV advanced stage BC | Two-arm RCT; (N) 150; (n=75): RT and C; (n=75): SK and P and C | 20 min of SK and P on an everyday basis for 6 months taught by a trained yoga teacher and 3 days of contact workshop. | Cortisol (serum) nmg/L | Mean and SD of SK and P group vs. Reg. T, at baseline 421±70 vs. 493±51; at 3rdmonth (341.4±51.4 vs. 549.2±69.5 ng/L) and at the 6thmonth’s (376.2±74.9 vs. 517.8±69.7 ng/L). |
| Bower et al. |
2014; USA | Study participants: 54±5.4 | Stages 0-II BC survivors | Two-arm RCT; (N) 31; (n=15): Health Education; (n=16): Iyengar Yoga | 90 min Iyengar yoga, twice weekly for 12 weeks, administered by a certified instructor. | TNF-RII (pg./mL); IL-1 RA (pg./mL); IL-6 (pg./mL); CRP mg/L; Cortisol (saliva) (nmol/L). |
In Iyengar Yoga TNF-RII was significant, P<0.05, but not significant in IL-1RA (P=0.16), IL-6 and CRP and cortisol. Follow-up at 3 months, no difference reported. |
| Chandwani et al. |
2014; USA | Waitlist: 52.11±1.34 Stretch: 51.4±1.32 Iyengar Yoga: 52.38±1.35 |
Stages 0-III Undergoing Radiotherapy | Three-arm RCT; (N) 164; (n=54): waitlist; (n=56): Stretching; (n=54): Integrated Yoga | 60 min of Integrated yoga program, one-on-one, thrice a week for 6 weeks administered by VYASA trained teacher. | Cortisol (saliva) nmol/L | Mean cortisol level at the end of radiotherapy: Yoga vs. stretch and WL (P=0.027 and P=0.008), respectively, and at 1 month yoga vs. WL (P=0.05 and P=0.04) Follow-up at 3 months, no difference reported. |
| Kiecolt- Glaser et al. |
2014; USA | Waitlist: 51.3±8.7 Hatha Yoga: 51.8±9.8 |
Stages 0 to III BC survivors | Two-arm RCT; (N) 200; (n=100): Waitlist; (n=100): Hatha Yoga | 90 min Hatha yoga, per session, twice weekly for 12 weeks administered by a Senior Yoga teacher. | IL-6 (pg./mL); IL -1β (pg./mL); TNF-α |
Immediate to post-intervention: IL-6 (P>0.027), TNF-α(P=0.027) and IL-β(P=0.037). Follow-up at 3 months: IL-6 (P=0.01), IL-1β(P=0.03), TNF-α(P>0.05). |
| Parma et al. |
2015; USA | EC: 54.4±7.0 CE: 57.6±6.6 Yoga: 56.7±9.6 | Breast cancer survivors | Three-arm RCT; (N) 94; (n=32): Ex of choice; (n=31): Comp. Ex; (n=31): Yoga Exercise | 60 min thrice weekly for 6 months for yoga-based exercise by a yoga instructor; Comprehensive exercise by a clinically Trained physical therapist. | IL-6 (pg./mL); IL-8 (pg./mL); TNF-α (pg./mL); CRP (μg./mL) |
Mean and SD (pre minus post-intervention); Yoga, CE and EC; respectively: IL-6 (35.49±2353.78; 317.62±3373.67; 557.28±2558.23) IL-8 (141.77±579.25; −52.10±454.40; −63.55±516.38) TNFα(184.61±750.28; 860.74±1620.38; −212.13±756.10) CRP (0.018±7.77; −0.37±12.90; 0.20±12.11). |
| Rao et al. | 2017; India | E&S therapy: 50.2±9.2 Integrated yoga: 48.9±9.1 | Metastatic breast cancer | Two-arm RCT; (N) 91; (n=46): E&S therapy.; (n=45): Integrated yoga | Twice weekly for 12 weeks in person by a physician in naturopathy and yoga and a yoga therapist. | Cortisol (saliva) | Mean and SD of pre-and post-intervention Yoga: 0.15±0.11, 0.11±0.13; Control: 0.19±0.25, 0.18±0.20 ES=0.4 |
AR: Adjuvant radiotherapy, SC and Ex: Supportive counselling and Exercise, HE: Health education, RT and C: Regular treatment with counselling, IY: Integrated yoga, S. Therapy: Supportive therapy, N and Y: Naturopathy and Yoga, Ex: Exercise, EC: Exercise of choice, CE: Comprehensive exercise, EST: Education and supportive therapy, SK: Sudarshan Kriya, P: Pranayama, VYASA: Swami Vivekananda Yoga Anusandhana Samsthana, IL: Interleukin, TNF: Tumour necrosis factor, TNF-RII: Tumour necrosis factor type two, IL-1RA: Interleukin-1 receptor antagonist, CRP: C-reactive protein, vs.: Versus
Intervention characteristics
YI consists of Iyengar yoga,[27,29] integrated yoga,[25,26,30,33] Hatha Yoga,[31] Sudarshan kriya[28] and yoga exercise,[32] the duration ranged from 4 weeks to 26 weeks, with each session time ranging from 30 min to 90 min. All the YI(s) were administered by certified yoga instructors or yoga therapists. The YI was effective on inflammatory biomarkers, TNF-alpha, and cortisol levels. There was no change in TNF receptor type II, IL-1 receptor antagonist, IL-6, IL-8, and C-reactive protein from baseline to post YI [Table 2].
Table 2:
Effect of yoga intervention on inflammatory biomarkers and cortisol.
| Parameters | Comparisons | Effect estimate | |
|---|---|---|---|
| MD | 95% CI for MD | ||
| Cortisol (log10) ng/dL (Banasik et al., 2011) | Yoga vs. control | −0.18 | −0.31–−0.04* |
| Cortisol (log10) ng/dL (Banasik et al., 2011) | Baseline vs. post-yoga | 0.17 | 0.08–0.26* |
| TNF receptor type II (pg/mL) (Bower et al., 2014) | Baseline vs. Post-yoga | 73 | −118.81–264.81 |
| TNF-α(pg/mL) (Parma et al., 2015) | Baseline vs. post-yoga | −147.6 | −991.83–−696.63* |
| IL-1 receptor antagonist (pg/mL) (Bower et al., 2014) | Baseline vs. post-yoga | −10 | −76.18–56.18 |
| IL-6 (pg/mL) (Bower et al., 2014) | Baseline vs. post-yoga | −0.16 | −0.65–0.33 |
| IL-6 (pg/mL) (Parma et al., 2015) | Baseline vs. post-yoga | −35 | −1883.8–1812.8 |
| IL-8 (pg/mL) (Parma et al., 2015) | Baseline vs. post-yoga | −173.8 | −551.87–204.27 |
| CRP (mg/L) (Bower et al., 2014) | Baseline vs. post-yoga | 0.63 | −3.04–4.30 |
| CRP (μg/mL) (Parma et al., 2015) | Baseline vs. post-yoga | −0.02 | −3.61–3.57 |
| Cortisol levels (nmoL/L) (Bower et al., 2014) | Baseline vs. post-yoga | −1.99 | −3.63–−0.35* |
| Cortisol levels (Parma et al., 2015) | Baseline vs. post-yoga | 0.04 | 0.01–0.07* |
| Salivary cortisol levels (Banasik et al., 2011) | Baseline vs. post-yoga | 0.07 | 0.02–0.12* |
MD: Mean difference, CI: Confidence interval, vs.: Versus, *: Significant
DISCUSSION
Six studies have determined the cortisol level; five using salivary samples[26,27,29,30,33] and one with serum samples.[28] Four out of nine studies had assessed inflammatory markers,[25,29,31,32] and all were pro-inflammatory markers, namely TNF-α,[25,31,32] TNF-RII,[29] IL-6,[29,31,32] IL-8,[32] IL-1β[31] and CRP.[29,32]
Stress hormone
Cortisol, a stress hormone secreted by the adrenal cortex, has a protective role during acute stress but a deleterious effect in chronic stress[19] by inhibiting the cells of the immune system.[36] Clinical and molecular research reveals a correlation between BC progression and a surge of cortisol.[13] Daytime cortisol anomaly in advanced BC is likely to die early than those with a usual level of cortisol (Stanford University report, June 2000). Six studies had appraised the YI effect on the cortisol level, five with samples of saliva and one with serum samples. Five out of six studies have shown a downtrend in cortisol levels,[26-28,30,33] and only one study reported no change in the cortisol level post-YI.[29]
Tumour necrosis factor
This inflammatory cytokine is released by the activated monocytes, macrophages, natural killer cells and other cells, such as B cells, T cells and fibroblasts.[37] Macrophages exert their function by binding to TNF-RII and its expression escalates in cancer, especially tumour cell proliferation in BC.[38] In our review, post-YI TNF-RII level was found stable but a surge was seen in the control group[29] and a downslide of TNF-a was noted, post-integrated yoga and yoga-based exercise.[25,32]
IL-6 and IL-8
IL-6 and IL-8 are cytokines released by the epithelial cells of the lungs and involve in tumour progression through angiogenesis,[39] their concentration correlates with the clinical stage of the disease and can be established for both prognosis and diagnosis of BC.[40] One out of three studies on IL-6 was found to be significant (P=0.01),[31] whereas a study conducted on IL-8 was not significant post-YI.[32]
IL1β
A pro-inflammatory biomarker, IL-1b, a family ligand of IL-1, is released by the activated macrophages and stimulates the progression of cancer in the tumour environment.[41] Its expression in BC has been recognised as a potential marker for increased risk of bone metastasis.[11] Post-hatha yoga IL-1b level was found to have dwindled.[31]
CRP
An inflammatory marker primarily released by hepatocytes and is linked with poor prognosis if significantly elevated;[42] two studies evaluated the CRP and found no significant change between the YI and the control groups.[29,32]
Long-term outcome
Three studies reported follow-up at 3 months. Post-hatha yoga showed a positive effect on IL-6 and IL-1b.[31] No significant change was reported post-Iyengar yoga on sTNF=RII, IL-IRA, IL-6, CRP and cortisol,[29] and post-integrated yoga programme on cortisol.[30]
Limitations
Stratification of BC cases into YI and control was reported among the qualified studies following the eligibility criteria. The heterogeneity in type and grade of BC, the YI procedures in terms of the number of days of practice, duration and training received, performance bias 89% unclear and attrition rate with 33% of the included studies; were the major limitations and concerns of this review. There was no comparison of longitudinal data on inflammatory biomarkers followed by YI in this review. Data on types of YI and their comparisons have not been studied in this review.
CONCLUSION
So far to our understanding, this is the only systematic review that is carried out focusing on the inflammatory markers, giving an insight into YI effect in the cancer microenvironment in BC cases. YI is found to have a beneficial effect on the cortisol level, the stress marker associated with BC progression. Pro-inflammatory markers namely tumour necrosis factor-alpha, IL-6 and IL-1b exhibited a favourable response unlike IL-8 and CRP. YI can be considered a supportive therapy to alleviate stress and inflammatory markers but does not guarantee the utility of YI as an alternative therapy for BC management. Conducting more studies that include anti-inflammatory and pro-inflammatory biomarkers as an outcome measure and a long-term follow-up may enable us to precisely gauge the YI effect in the cancer microenvironment.
PRISMA 2020 Main Checklist
Risk of Bias Assessment of each study
| Authors | Bias | Judgements | Justification |
|---|---|---|---|
| Rao et al., 2009 | Random sequence generation (selection bias) | Low risk | Randomised using random numbers generated by a random number table |
| Allocation concealment (selection bias) |
Low risk | Randomisation was performed using opaque envelopes with group assignments, which were opened sequentially in the order of assignment during recruitment. | |
| Blinding of participants (performance bias) |
Unclear risk | Authors specified that it was not possible to mask the yoga intervention from the subjects as yoga is a popular practice. |
|
| Blinding of outcome assessment (detection bias) | High risk | No adequate information, it only states the investigators (treating surgical oncologists) were blinded to the intervention. | |
| Incomplete outcome data (attrition bias) |
Low risk | N=98 at baseline, for analysis (n=33), (n=36) for yoga and control group, respectively. | |
| Selective bias (reporting bias) | Low risk | No registered protocol information, but no clue on the selective bias. | |
| Other bias | Unclear risk | The findings of IFN-gamma and soluble IL-2R alpha were narratively reported as no significant change. | |
| Vadiraja et al., 2009 | Random sequence generation (selection bias) | Low risk | Randomised using computer-generated random numbers. |
| Allocation concealment (selection bias) |
Low risk | Randomisation was performed using opaque envelopes with group assignments. Personnel who had no part in the trial performed randomisation. | |
| Blinding of participants (performance bias) |
High risk | No information on blinding the participants. | |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on the outcome assessor. | |
| Incomplete outcome data (attrition bias) |
High risk | Attrition of 5% and 25% in yoga and control groups, respectively. | |
| Selective bias (reporting bias) | Low risk | No protocol registered, no indication of reporting bias | |
| Other bias | Low risk | No clue about other biases. | |
| Banasik et al., 2011 United States |
Random sequence generation (selection bias) | Unclear risk | No information on the randomisation process. |
| Allocation concealment (selection bias) |
Unclear risk | No information on allocation concealment. | |
| Blinding of participants (performance bias) |
Unclear risk | No information on the blinding of participants. | |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding of outcome assessor. | |
| Incomplete outcome data (attrition bias) |
Low risk | Though attrition rate is 22%. Baseline n=18, post-intervention n=14 (7 per group), so it is balanced between groups. | |
| Selective bias (reporting bias) | Low risk | No indication of selective reporting. | |
| Other bias | Unclear risk | There is a large difference in baseline between groups. | |
| Neeta et al., 2013 | Random sequence generation (selection bias) | Low risk | Generated random allocation numbers, by an independent team. |
| Allocation concealment (selection bias) |
Low risk | The independent team generated random allocation numbers; separate staff imparted allocation into the control versus intervention arm by opening opaque envelopes containing allocation information in the subsequent recruitments. | |
| Blinding of participants (performance bias) | Unclear risk | No information on the blinding of participants. | |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding is followed at the blood sample collection, processing, testing and analysis levels. A separate team worked on these components and used only codes, which were broken at the time of the final analysis by the statistician team | |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on attrition | |
| Selective bias (reporting bias) | Low risk | No information on registered protocol but no reporting bias was identified. | |
| Other bias | Low risk | None identified | |
| Bower et al., 2014 | Random sequence generation (selection bias) | Low risk | The allocation sequence was generated independently by the study statistician. |
| Allocation concealment (selection bias) |
Low risk | Allocation is concealed in opaque envelopes. | |
| Blinding of participants (performance bias) | Unclear risk | Participants were not aware of the study hypothesis but were aware of the group assigned. | |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessors for the performance tasks were blinded to group assignment | |
| Incomplete outcome data (attrition bias) | Low risk | Out of N (31) at 3 months follow-up n=29 inflammatory markers; n=28 salivary cortisol. | |
| Selective bias (reporting bias) | Low risk | Study protocol registered (Cortisol not specified). Reporting bias not identified. | |
| Other bias | Low risk | None identified | |
| Chandwani et al., 2014 |
Random sequence generation (selection bias) | Low risk | Adaptive randomisation was adopted. Stratified by stage of cancer and treated with radiotherapy or not. |
| Allocation concealment (selection bias) |
Unclear risk | Inadequate information on allocation concealment. | |
| Blinding of participants (performance bias) | Unclear risk | Control participants were told not to practice yoga during the study period (chances of intervention contamination). | |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding, though unlikely to influence the outcome. | |
| Incomplete outcome data (attrition bias) | Low risk | Loss of follow-up differs at 1-, 3-and 6-month post-intervention but is balanced between groups. | |
| Selective bias (reporting bias) | Low risk | The protocol is not available but no indication of reporting bias. | |
| Other bias | Low risk | None identified. | |
| Kiecolt-Glaser et al., 2014 |
Random sequence generation (selection bias) | Low risk | Online randomisation by the data manager. |
| Allocation concealment (selection bias) |
Low risk | The data manager has no contact with the participants. | |
| Blinding of participants (performance bias) | Unclear risk | Participants knew the assigned group. | |
| Blinding of outcome assessment (detection bias) | Low risk | Participants were told not to reveal the assigned group to the assessor. | |
| Incomplete outcome data (attrition bias) | Low risk | 15% attrition was considered in sample size calculation (though 4% and 0% attrition in yoga and control group, respectively). | |
| Selective bias (reporting bias) | Low risk | Protocol registered. No clue about the bias. | |
| Other bias | Low risk | None detected | |
| Parma et al., 2015 | Random sequence generation (selection bias) | Low risk | Randomisation technique minimisation adaptive was mobilised. |
| Allocation concealment (selection bias) |
Low risk | Using a minimisation adaptive randomisation technique, participant covariates of age, body mass index (BMI) and cardiorespiratory capacity (estimated VO2 max) | |
| Blinding of participants (performance bias) | Unclear risk | No information on the blinding of participants | |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information on blinding outcome assessment | |
| Incomplete outcome data (attrition bias) | High risk | 24% attrition with yoga constituting 12%, the other two groups 6% each | |
| Selective bias (reporting bias) | Low risk | No information on registered protocol but reporting bias was not identified. | |
| Other bias | High risk | Imbalance of sample between groups, post-intervention. | |
| Rao et al., 2017 | Random sequence generation (selection bias) | Low risk | Randomised using random numbers generated by a random number table. |
| Allocation concealment (selection bias) |
Low risk | A person who had no part in the trial randomly allocated consenting participants to random numbers generated by a random number table at a different site. | |
| Blinding of participants (performance bias) | Unclear risk | Authors specified that yoga being a popular intervention, it was not possible to mask the yoga intervention from the subjects. | |
| Blinding of outcome assessment (detection bias) | Low risk | The saliva and blood samples were blinded by the technicians who analysed the coded samples at a site different from the study centre. | |
| Incomplete outcome data (attrition bias) | High risk | 50% attrition, on salivary samples | |
| Selective bias (reporting bias) | Low risk | No registered protocol information, but no clue on the selective bias. | |
| Other bias | Low risk | None identified |
Funding Statement
Financial support and sponsorship
Nil.
Footnotes
How to cite this article: Kaje KC, Dsilva F, Sanal TS, Latha T, Kumar S, D’Souza C. Effect of Yoga intervention on inflammatory biomarkers among women with breast cancer – A systematic review. Indian J Palliat Care 2023;29:223-33.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Salehiniya H, Ghoncheh M, Pournamdar Z. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43–6. doi: 10.7314/APJCP.2016.17.S3.43. [DOI] [PubMed] [Google Scholar]
- 3.Schick J, Ritchie RP, Restini C. Breast cancer therapeutics and biomarkers: Past, present, and future approaches. Breast Cancer Basic Clin Res. 2021;15:1–19. doi: 10.1177/1178223421995854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst. 2019;111:1298–306. doi: 10.1093/jnci/djz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ICMR NCDIR Report of National Cancer Registry Programme 2020. A scientific way to understand about Cancer. The Report of National Cancer Registry Programme. 2020. Available from: https://ncdirindia.org https://www.ncdirindia.org/All_Reports/Report_2020/default.aspx.
- 6.Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate : A 25-year study. Asian Pac J Cancer Prev. 2019;20:2015–20. doi: 10.31557/APJCP.2019.20.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemi SM, Rafiemanesh H, Aghamohammadi T, Badakhsh M, Amirshahi M, Sari M, et al. Prevalence of anxiety among breast cancer patients : A systematic review and meta-analysis. Breast Cancer. 2019;27:1–13. doi: 10.1007/s12282-019-01031-9. [DOI] [PubMed] [Google Scholar]
- 8.Hoge EA, Bui E, Palitz SA, Schwarz NR, Owens ME, Johnston JM, et al. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. HHS Public Access. 2019;262:328–32. doi: 10.1016/j.psychres.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: Relationship to glucocorticoids. Brain Behav Immun. 2007;21:251–8. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard LC, Antoni MH, Blomberg BB, Stagl JM, Gudenkauf LM, Jutagir DR, et al. Postsurgical depressive symptoms and proinflammatory cytokine elevations in women undergoing primary treatment for breast cancer. Psychosom Med. 2016;78:26–37. doi: 10.1097/PSY.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulotta C, Ottewell P. The role of IL-1B in breast cancer bone metastasis. Endocr Relat Cancer. 2018;25:R421–34. doi: 10.1530/ERC-17-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iles KE, Dickinson DA. International Encyclopedia of Public Health. Amsterdam, Netherlands: Elsevier; 2016. Carcinogens, Environmental, 2nd ed, Vol. 1; pp. 422–37. [DOI] [Google Scholar]
- 13.Al Sorkhy M, Fahl Z, Ritchie J. Cortisol and breast cancer: A review of clinical and molecular evidence. Ann Cancer Res Ther. 2018;26:19–25. doi: 10.4993/acrt.26.19. [DOI] [Google Scholar]
- 14.Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35:1–16. doi: 10.1089/jir.2014.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2021;30(Suppl):S19–25. doi: 10.1016/j.bbi.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosain R, Gage-Bouchard E, Ambrosone C, Repasky E, Gandhi S. Stress reduction strategies in breast cancer: Review of pharmacologic and nonpharmacologic based strategies. Semin Immunopathol. 2020;42:719–34. doi: 10.1007/s00281-020-00815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2015;62:1377–84. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 18.Greenlee H, Balneaves LG, Carlson LE, Cohen M, Deng G, Hershman D, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J Natl Cancer Inst Monogr. 2014;2014:346–58. doi: 10.1093/jncimonographs/lgu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lengacher CA, Reich RR, Paterson CL, Shelton M, Shivers S, Ramesar S, et al. A large randomized trial: Effects of mindfulness-based stress reduction (MBSR) for breast cancer (BC) survivors on salivary cortisol and IL-6. Biol Res Nurs. 2019;21:39–49. doi: 10.1177/1099800418789777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors : A systematic review and meta-analysis. BMC Cancer. 2012;12:412. doi: 10.1186/1471-2407-12-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaje KC, Dsilva F, Latha T, Sanal T, D'Souza C, Kumar S. A systematic review protocol on the effect of yoga intervention on inflammatory biomarkers among women with breast cancer. J Health Allied Sci NU. 2022;12:e1–5. doi: 10.1055/s-0042-1755583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802. doi: 10.1002/14651858.CD010802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anestin AS, Dupuis G, Lanctôt D, Bali M. The effects of the bali yoga program for breast cancer patients on chemotherapy-induced nausea and vomiting: Results of a partially randomized and blinded controlled trial. J Evid Based Complement Alternat Med. 2017;22:721–30. doi: 10.1177/2156587217706617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV. Hoboken, New Jersey: Wiley; 2008. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 25.Rao RM, Nagendra HR, Raghuram N, Vinay C, Chandrashekara S, Gopinath KS, et al. Influence of yoga on postoperative outcomes and wound healing in early operable breast cancer patients undergoing surgery. Int J Yoga. 2008;1:33–41. doi: 10.4103/0973-6131.36795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vadiraja HS, Raghavendra RM, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: A randomized controlled trial. Integr Cancer Ther. 2009;8:37–46. doi: 10.1177/1534735409331456. [DOI] [PubMed] [Google Scholar]
- 27.Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23:135–42. doi: 10.1111/j.1745-7599.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar N, Bhatnagar S, Velpandian T, Patnaik S, Menon G, Mehta M, et al. Randomized controlled trial in advance stage breast cancer patients for the effectiveness on stress marker and pain through Sudarshan kriya and pranayam. Indian J Palliat Care. 2013;19:180–5. doi: 10.4103/0973-1075.121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–9. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandwani KD, Perkins G, Nagendra HR, Raghuram NV, Spelman A, Nagarathna R, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058–65. doi: 10.1200/JCO.2012.48.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, et al. Yoga's impact on inflammation, mood, and fatigue in breast cancer survivors: A randomized controlled trial. J Clin Oncol. 2014;32:1040–9. doi: 10.1200/JCO.2013.51.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parma DL, Hughes DC, Ghosh S, Li R, Treviño-Whitaker RA, Ogden SM, et al. Effects of six months of Yoga on inflammatory serum markers prognostic of recurrence risk in breast cancer survivors. Springerplus. 2015;4:143. doi: 10.1186/s40064-015-0912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao RM, Vadiraja HS, Nagaratna R, Gopinath KS, Patil S, Diwakar RB, et al. Effect of yoga on sleep quality and neuroendocrine immune response in metastatic breast cancer patients. Indian J Palliat Care. 2017;23:253–60. doi: 10.4103/IJPC.IJPC_102_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RevMan 5 download RevMan. Available from: https://www.cc-ims.net/revman [Last accessed on 2022 Apr 17]
- 35.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. Available from: https://www.handbook.cochrane.org [Last accessed on 2022 Apr 20]
- 36.Sudheimer KD, O'Hara R, Spiegel D, Powers B, Kraemer HC, Neri E, et al. Cortisol, cytokines, and hippocampal volume interactions in the elderly. Front Aging Neurosci. 2014;6:153. doi: 10.3389/fnagi.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine SJ, Larivee P, Logun C, Angus CW, Ognibene FP, Shelhamer JH. Tumor necrosis factor-a induces mucin hypersecretion and MUC-2 gene expression by human airway epithelial cells. Am J Respir Cell Mol Biol. 1995;12:196–204. doi: 10.1165/ajrcmb.12.2.7865217. [DOI] [PubMed] [Google Scholar]
- 38.Mercogliano MF, Bruni S, Elizalde PV, Schillaci R. Tumor necrosis factor a blockade: An opportunity to tackle breast cancer. Front Oncol. 2020;10:584. doi: 10.3389/fonc.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shastri MD, Stewart N, Horne J, Peterson GM, Gueven N, Sohal SS, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10:e0126763. doi: 10.1371/journal.pone.0126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–4. [PubMed] [Google Scholar]
- 41.Gelfo V, Romaniello D, Mazzeschi M, Sgarzi M, Grilli G, Morselli A, et al. Roles of il-1 in cancer: From tumor progression to resistance to targeted therapies. Int J Mol Sci. 2020;21:6009. doi: 10.3390/ijms21176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart PC, Rajab IM, Alebraheem M, Potempa LA. C-reactive protein and cancer-diagnostic and therapeutic insights. Front Immunol. 2020;11:595835. doi: 10.3389/fimmu.2020.595835. [DOI] [PMC free article] [PubMed] [Google Scholar]