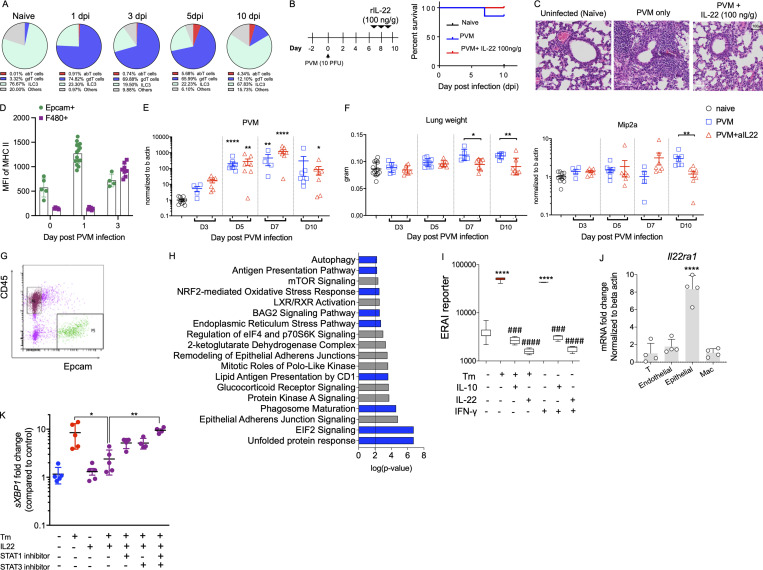
Figure S3.
Late IL-22–mediated suppression of epithelial MHC II does not alter pathology in viral infection. (A) Source of IL-22 was assessed in naïve animals or animals with PVM infection; flow cytometric relative data presented as percentages. abT, αβ T cells; gdT, γδ T cells. (B) Schematic experimental diagram and survival curve. (C) Representative H&E staining images of the lungs of WT mice infected with PVM (100 PFU) at day 0 and treated with rIL-22 (100 ng/mouse) on days 6, 8, and 10 prior to analysis at day 10. (D) MFI of MHC II on Epcam+ve cells or F4/80+ve cells during PVM infection. (E) Relative expression of PVM-sh assessed by qRT-PCR. (F) Lung weight and chemokine, Mip-2a, gene expression level at indicated day in the α-IL22 antibody (12.5 μg/mouse) treated mice as in A. (G) Representative flow cytometric plot showing FACS-sorted Epcam cells for RNA-Seq analysis from WT animals infected with PVM (10 PFU) with and without α-IL22-antibody treatment, on day 3 after infection. (H) DAVID gene ontology analysis showing the upregulation of ER stress, antigen presentation and the unfolded protein response pathways in the EpCAM+ve cells from animals treated with α-IL22-antibody. Data are presented as mean ± SEM (n = 6–7). (I) Fluorescence of ER stress activated indicator (ERAI) reporter transfected in LS174T cells treated with Tm (10 µg/ml), IL-10 (50 ng/ml), IL-22 (50 ng/ml), or IFNγ (50 ng/ml), alone or in combination for 24 h. (J) The relative expression level of IL-22ra1 mRNA was measured in FACS-sorted epithelial cells (Epcam+), endothelial cells (CD31+), T cells (CD3+), and macrophages (Cd11b+) from naïve C57BL/6 mice. (K) HBECs treated with Tm (1 µg/ml), IL-22 (10 ng/ml) alone, or in combination in the presence of 50 µmol/liter of STAT1 and STAT3 inhibitors and sXBP1 expression was assessed by qRT-PCR. Data are representative of two independent experiments and presented as mean ± SEM (n = 4–10). One-way ANOVA, Bonferroni’s post hoc test; *P < 0.05, **P < 0.01, and ****P < 0.0001. ###P < 0.001 and ####P < 0.0001 compared to cellular stressors Tm and IFNγ.