Abstract
Pre-existing tetracycline resistance in Neisseria gonorrhoeae limits the effectiveness of post-exposure prophylaxis (PEP) with doxycycline against gonorrhea, and selection for tetracycline resistance may influence prevalence of multi-drug resistant strains. Using genomic and antimicrobial susceptibility data from N. gonorrhoeae, we assessed the near-term impact of doxycycline PEP on N. gonorrhoeae resistance.
Pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) with doxycycline has been shown to decrease rates of bacterial sexually transmitted infections (STIs) in clinical trials [1–4], and doxycycline PEP is already used by some men who have sex with men (MSM) [5] and is now recommended by several public health departments in California, including in San Francisco, Santa Clara County, and Alameda County [6–8]. Official guidelines from the US Centers for Disease Control and Prevention (CDC) and other public health institutions in the United States are pending. However, the British Association for Sexual Health and HIV and Public Health England do not currently recommend doxycycline PEP due to the risk of antimicrobial resistance in N. gonorrhoeae and other bacterial pathogens [9].
Pre-existing tetracycline resistance in the N. gonorrhoeae population resulted in lower doxycycline PEP effectiveness against gonorrhea than against syphilis and chlamydia [2] and reflects tetracycline and multidrug resistant strains of N. gonorrhoeae that may be selected for by doxycycline PEP, including strains resistant to current first line treatment. Given this concern, we evaluated the potential near-term impacts of doxycycline PEP on antimicrobial resistance in N. gonorrhoeae using whole genome sequencing (WGS) data and minimum inhibitory concentrations (MICs) from a global collection of 5644 N. gonorrhoeae isolates (Supplementary Tables 1 and 2), including 1041 isolates from 2018 collected and sequenced by CDC's Gonococcal Isolate Surveillance Program (GISP) [10].
Interpretative breakpoints for N. gonorrhoeae susceptibility and resistance to doxycycline have not been defined; however, treatment failures have been observed when isolates have doxycycline MICs ≥ 1 µg/mL [11], and doxycycline MICs correlate with tetracycline MICs [12]. Tetracycline resistance can be mediated by plasmid-encoded tetM, which confers high-level resistance, and chromosomally encoded mutations in rpsJ, porB, and the mtr operon [13]. We excluded isolates that had tetracycline MICs that most likely represented reporting errors (Supplementary Text).
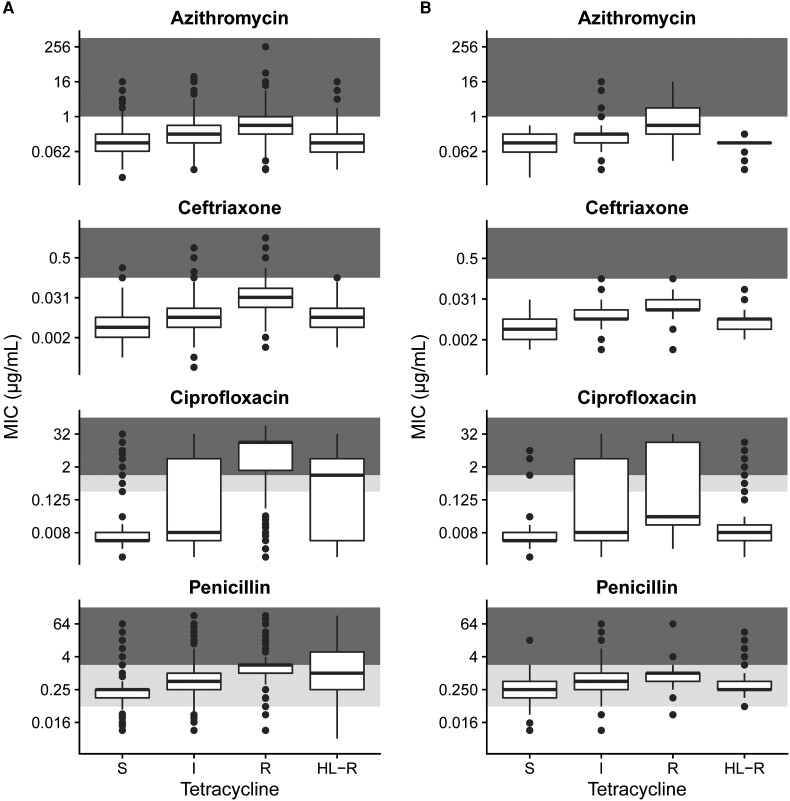
We found that co-resistance to other antimicrobials was most common in isolates with chromosomally encoded tetracycline resistance (Figure 1). Ceftriaxone, azithromycin, and ciprofloxacin MICs were significantly higher in isolates with tetracycline MICs of 2–8 µg/mL compared to isolates not resistant to tetracycline and to isolates with high-level tetracycline resistance (P < .0001, Mann-Whitney test). Ceftriaxone reduced susceptibility (MIC ≥ 0.125 µg/mL) was rare in GISP isolates (n = 2), but the global data suggested that reduced susceptibility is most often acquired by strains with chromosomally mediated tetracycline resistance, including internationally transmitting strains encoding the penA 60 allele, which confers ceftriaxone reduced susceptibility [14, 15]. Azithromycin resistance appeared in 23.5% (56/238) of GISP isolates resistant to tetracycline; all that were tetracycline and azithromycin co-resistant had chromosomally encoded tetracycline resistance. Ciprofloxacin resistance appeared in 33.6% (80/238) of GISP isolates resistant to tetracycline; however, only 12.9% (13/101) of isolates with tetM were also ciprofloxacin resistant. Recent work analyzing the presence of resistance-associated alleles in N. gonorrhoeae genomes sequenced from EuroGASP showed that lineages encoding tetM had fewer additional resistance alleles than lineages encoding tetracycline-resistance associated alleles of rpsJ [16], suggesting that we may expect a similar impact of doxycycline PEP on antimicrobial resistance in the United States and Europe.
Figure 1.
Co-resistance with other antimicrobials is highest among isolates with chromosomally encoded resistance to tetracyclines. Isolates were classified as susceptible (MIC ≤ 0.25 µg/mL), intermediate (0.25 < MIC < 2 µg/mL), resistant (2 ≤ MIC ≤ 8 µg/mL), or high-level resistant (MIC > 8 µg/mL) in 5644 global Neisseria gonorrhoeae isolates (A) and 1041 isolates collected in the United States in 2018 (B). Background shading corresponds to susceptible (white), intermediate (light gray), and resistant/non-susceptible (dark gray) MICs for each antimicrobial. Abbreviation: MIC, minimum inhibitory concentration.
Doxycycline PEP has primarily been studied in populations including MSM and transgender persons who have sex with men; preliminary results from a clinical trial in cisgender women have been reported, but the final results are pending [17]. The distribution of isolates classified as susceptible (MIC ≤ 0.25 µg/mL), intermediate (0.25 < MIC < 2 µg/mL), resistant (2 ≤ MIC ≤ 8 µg/mL), or high-level resistant (MIC > 8 µg/mL) significantly differed between MSM and men who have sex with women (MSW) (Supplementary Table 3, P < .0001, χ2 test). Although the proportion of isolates with tetracycline resistance was similar between MSM (26.8%, 91/340) and MSW (21.3%, 125/587), tetracycline susceptibility was more common among MSW (23.0%, 135/587) compared to MSM (10.3% 35/340). Among MSM, the majority of isolates (62.9%, 214/340) had intermediate tetracycline MICs. In addition to selection for resistant lineages in MSM populations, this large population of N. gonorrhoeae with intermediate MICs may represent a reservoir for rapid evolution of resistance. However, the evolutionary pathways and barriers to doxycycline resistance and their variation by genomic background in N. gonorrhoeae are unknown.
The composition of N. gonorrhoeae isolates was not homogeneous across the United States, and the percentage of tetracycline resistant isolates collected across Health and Human Services (HHS) regions ranged from 17.4% to 52.0% (Supplementary Figure 1). Mechanisms of resistance also varied, and high-level resistance was not evenly distributed across geographic regions, with the lowest proportion of isolates with high-level resistance in HHS regions from the east coast and southeastern United States (1.7%–4.4%). This indicates that doxycycline PEP will likely have regionally varying effectiveness and impact on circulating N. gonorrhoeae.
In our study, the genomic data representing the N. gonorrhoeae population in the United States were from isolates collected in 2018, and recent changes in treatment guidelines [18] and introductions of resistant lineages, such as isolates encoding penA 60 [19], may have shifted the landscape of antimicrobial resistance in the United States. The number of genomes meeting our quality thresholds from the southeastern United States was low (Supplementary Figure 1), so these data may not accurately capture lineages transmitting in this region. We emphasize that the long-term impacts of doxycycline PEP on the N. gonorrhoeae population in the United States are unknown, as circulating lineages may acquire new resistance alleles [20–22]. Additionally, epidemic spread of N. gonorrhoeae with high-level tetracycline resistance has been described in other geographic regions, and introduction of these lineages to the United States is possible [23, 24]. For example, a recent study found that strains encoding tetM are highly prevalent among women in Kenya [25], and phylogenetic analysis of our global dataset identified several lineages with high-level tetracycline resistance not represented among GISP isolates (Supplementary Figure 2).
The near-term impact of doxycycline PEP on N. gonorrhoeae antimicrobial resistance and circulating lineages will be influenced by the strength of selection for plasmid-encoded tetracycline resistance compared to chromosomally-encoded tetracycline resistance. If doxycycline PEP use selects for all tetracycline resistant lineages, there is potential for increased resistance to other antimicrobials as well. However, if doxycycline PEP use primarily selects for lineages with tetM-mediated resistance, we may observe a temporary decline in resistance to other antimicrobials due to relatively less co-resistance in these lineages. Timely genomic surveillance, with a focus on doxycycline PEP users, is needed to distinguish between these outcomes and to detect the emergence of novel combinations of resistance-associated alleles.
METHODS
Data Set
Whole genome sequencing data and minimum inhibitory concentrations were collected from previously published N. gonorrhoeae genomic studies (Supplementary Table 1). Isolates were included if the tetracycline MIC was reported with sufficient precision to categorize isolates as susceptible, intermediate, resistant, or high-level resistant.
Genome Assembly
Whole genomes sequencing data were assembled as previously described [26]. Briefly, we used SPAdes v 3.12.0 [27] for de novo assembly; the –careful flag was used to correct assemblies, and contigs were removed if coverage was <10 × or contig length was <500 nucleotides. Reads were additionally mapped to the NCCP11945 reference genome (NC_011035.1) using BWA-MEM v 0.7.17 [28], and variants were called using Pilon v 1.23 [29] with a minimum mapping quality of 20 and 10 × minimum coverage.
Quality Control of Genomic Data
We included isolates with genomes meeting the following quality control filters: the number of contigs was <500, the assembly length and number of annotated genes was expected for N. gonorrhoeae, coverage was >30×, at least 80% of reads mapped to the N. gonorrhoeae reference genome, and fewer than 12% of sites in the N. gonorrhoeae reference genome were unable to be confidently called using our assembly pipeline. Quality control metrics for included genomes is summarized in Supplementary Table 2. Additionally, we required that the contig encoding tetM was >10 000 nucleotides in length and at least 15 × coverage.
Phylogenetic Analysis
We used pseudogenomes derived from reference mapping and variant calls for phylogenetic analysis; we required that 90% of reads supported an allele to include the position in an isolate's pseudogenome. We used Gubbins v 2.4.1 [30] to identify recombinant regions and reconstruct the phylogeny.
Statistical Analysis
All statistical analysis was performed in R v 4.1.3 [31]. Significance of differences between MICs to ceftriaxone, azithromycin, ciprofloxacin, and penicillin was assessed using a Mann-Whitney test. Significance of differences of susceptible, intermediate, resistant, and high-level resistant isolates among demographic groups was assessed using a χ2 test.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Tatum D Mortimer, Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Yonatan H Grad, Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Notes
Author Contributions. T. D. M. performed data curation and analysis. Y. H. G. supervised and managed the study. T. D. M. and Y. H. G. wrote the manuscript, had full access to the data reported, and were responsible for the decision to submit the manuscript for publication.
Data availability. Data and code are available at https://github.com/gradlab/doxyPEP_genomics.
Disclaimer . The findings, conclusions, and views expressed are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases (grant numbers R01 AI132606 and R01 AI153521) and CDC contract number 200-2016-91779 to Y. H. G.
References
- 1. Bolan RK, Beymer MR, Weiss RE, Flynn RP, Leibowitz AA, Klausner JD. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high risk sex: a randomized, controlled pilot study. Sex Transm Dis 2015; 42:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molina J-M, Charreau I, Chidiac C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis 2018; 18:308–17. [DOI] [PubMed] [Google Scholar]
- 3. Luetkemeyer AF, Donnell D, Dombrowski JC, et al. Postexposure doxycycline to prevent bacterial sexually transmitted infections. N Engl J Med 2023; 388:1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molina J, Bercot B, Assoumou L, et al. ANRS 174 DOXYVAC: an open-label randomized trial to prevent STIs in MSM on PrEP [CROI Abstract 119]. In: Special Issue: Abstracts From CROI 2023 Conference on Retroviruses and Opportunistic Infections. 2023: 49. [Google Scholar]
- 5. Grant JS, Stafylis C, Celum C, et al. Doxycycline prophylaxis for bacterial sexually transmitted infections. Clin Infect Dis 2020; 70:1247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. HIV and STI Prevention | San Francisco City Clinic . Available at: https://www.sfcityclinic.org/providers/guidelines/hiv-and-sti-prevention. Accessed 18 April 2023.
- 7. Sexually Transmitted Infections—Public Health Providers—County of Santa Clara . Available at: https://publichealthproviders.sccgov.org/diseases/sexually-transmitted-infections. Accessed 18 April 2023.
- 8. Information to Healthcare Providers on Doxy-PEP . Available at: https://acphd.org/health-alerts/advisory/information-to-healthcare-providers-on-doxy-pep-feb-07-2023/. Accessed 18 April 2023.
- 9. Kohli M, Medland N, Fifer H, Saunders J. BASHH Updated position statement on doxycycline as prophylaxis for sexually transmitted infections. Sex Transm Infect 2022; 98:235–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reimche JL, Chivukula VL, Schmerer MW, et al. Genomic analysis of the predominant strains and antimicrobial resistance determinants within 1479 Neisseria gonorrhoeae isolates from the U.S. Gonococcal isolate surveillance project in 2018. Sex Transm Dis 2021; 48:S78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wiesner PJ, Holmes KK, Sparling PF, et al. Single doses of methacycline and doxycycline for gonorrhea: a cooperative study of the frequency and cause of treatment failure. J Infect Dis 1973; 127:461–6. [DOI] [PubMed] [Google Scholar]
- 12. Whittington WL, Roberts MC, Hale J, Holmes KK. Susceptibilities of Neisseria gonorrhoeae to the glycylcyclines. Antimicrob Agents Chemother 1995; 39:1864–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mortimer TD, Grad YH. Applications of genomics to slow the spread of multidrug-resistant Neisseria gonorrhoeae. Ann N Y Acad Sci 2019; 1435:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 2016; 60:4339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Day M, Pitt R, Mody N, et al. Detection of 10 cases of ceftriaxone-resistant Neisseria gonorrhoeae in the United Kingdom, December 2021 to June 2022. Euro Surveill 2022; 27:2200803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanbaelen T, Manoharan-Basil SS, Kenyon C. Doxycycline post exposure prophylaxis could induce cross-resistance to other classes of antimicrobials in Neisseria gonorrhoeae: an in-silico analysis. Sex Transm Dis 2023. [DOI] [PubMed] [Google Scholar]
- 17. Stewart J, Oware K, Donnell D, et al. Doxycycline postexposure prophylaxis for prevention of STIs among cisgender women [CROI Abstract 121]. In: Special Issue: Abstracts From CROI 2023 Conference on Retroviruses and Opportunistic Infections. 2023:49-50. [Google Scholar]
- 18. Cyr S S, Barbee L, Workowski KA, et al. Update to CDC's Treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Department of Public Health announces first cases of concerning gonorrhea strain | Mass.gov. Available at: https://www.mass.gov/news/department-of-public-health-announces-first-cases-of-concerning-gonorrhea-strain. Accessed 8 March 2023.
- 20. De Silva D, Peters J, Cole K, et al. Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect Dis 2016; 16:1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williamson DA, Chow EPF, Gorrie CL, et al. Bridging of Neisseria gonorrhoeae lineages across sexual networks in the HIV pre-exposure prophylaxis era. Nat Commun 2019; 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Golparian D, Vestberg N, Södersten W, et al. Multidrug-resistant Neisseria gonorrhoeae isolate SE690: mosaic penA-60.001 gene causing ceftriaxone resistance internationally has spread to the more antimicrobial-susceptible genomic lineage, Sweden, September 2022. Euro Surveill 2023; 28:2300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Dyck E, Laga M, Manoka AT, Behets F, Piot P. Epidemic spread of plasmid-mediated tetracycline resistant Neisseria gonorrhoeae in Zaire. Int J STD AIDS 1995; 6:345–7. [DOI] [PubMed] [Google Scholar]
- 24. Cehovin A, Harrison OB, Lewis SB, et al. Identification of novel Neisseria gonorrhoeae lineages harboring resistance plasmids in coastal Kenya. J Infect Dis 2018; 218:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soge OO, Issema R, Bukusi E, et al. Predominance of high-level tetracycline-resistant Neisseria gonorrhoeae in Kenya: implications for global implementation of doxycycline post-exposure prophylaxis for prevention of sexually transmitted infections. Sex Transm Dis 2023;50:317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortimer TD, Zhang JJ, Ma KC, Grad YH. Loci for prediction of penicillin and tetracycline susceptibility in Neisseria gonorrhoeae: a genome-wide association study. Lancet Microbe 2022; 3:e376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Computational Biol 2012; 19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:13033997 [q-bio] 2013; published online March 16. Available at: http://arxiv.org/abs/1303.3997. Accessed 29 September 2015.
- 29. Walker BJ, Abeel T, Shea T, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 2014; 9:e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Croucher NJ, Page AJ, Connor TR, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucl Acids Res 2015; 43:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2021Available at: https://www.R-project.org/. Accessed 12 December 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.