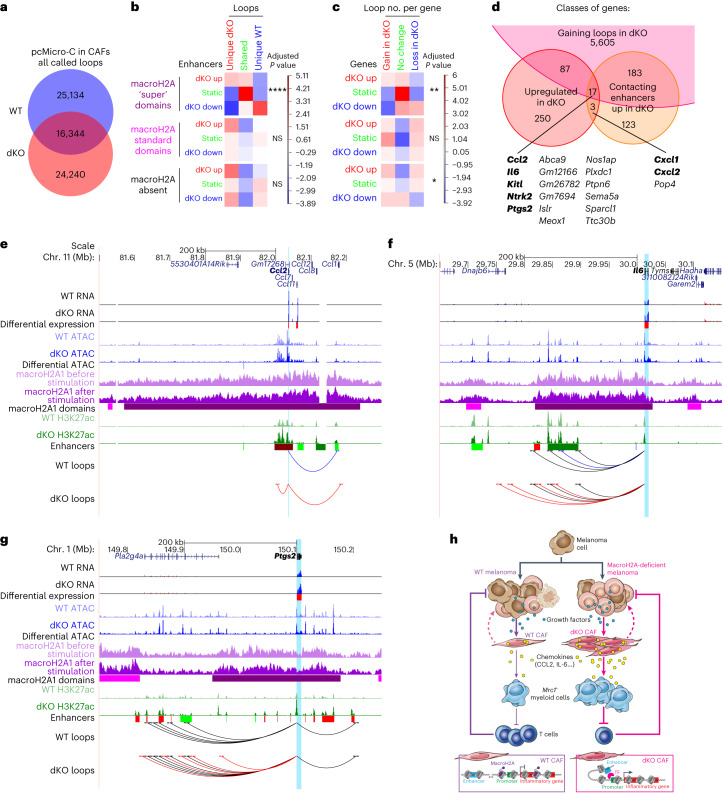
Fig. 7. DAEs and DEGs acquire changes in chromatin looping in dKO tumours.
a, Extent of overlap between chromatin loops detected in WT and dKO CAFs after serum stimulation at 10 kb resolution. b, Chi-square test of independence evaluating the association between changes in H3K27ac level at enhancers and gains or losses of loops to these enhancers in the absence of macroH2A. Combinations of loop and enhancer status are stratified according to the position of enhancers with respect to MCDs. c, As in b, but for changes in gene expression and in the total number of loops per gene. For b and c, P values adjusted for multiple comparisons shown: *P < 0.05, **P < 0.01, ****P < 0.0001. Exact P values are provided as numerical source data. d, Overlap between genes upregulated, in contact with enhancers gaining H3K27ac, and with net loop gains in dKO tumours. Genes in bold are associated with inflammatory signalling pathways according to HOMER analysis. e, University of California Santa Cruz (UCSC) genome browser screenshots of the Ccl2 locus and its chromatin environment showing indicated transcriptomic and epigenomic features. Bars under RNA-seq and ATAC–seq tracks indicate significantly upregulated (red) or downregulated (blue) genes or accessible regions in dKO versus WT sorted CAFs. Bars under macroH2A CUT&RUN tracks indicate ‘super’ (purple) and ‘standard’ (magenta) macroH2A chromatin domains. Below H3K27ac tracks, bright and dark bars indicate TEs and SEs, respectively; red, blue and green denote gain, loss and no change, respectively of H3K27ac level in dKO versus WT CAFs. Chromatin loops, originating at the promoter of the highlighted gene, are shown in red if specific for the dKO, blue for the WT, and black if shared. f, As in e, but for the Il6 locus. g, As in e, but for the Ptgs2 locus. h, Model of the impact of macroH2A loss on the melanoma TME. In the absence of macroH2A, inflammatory genes in CAFs become intrinsically hyperinducible owing to increased enhancer activity and promoter–enhancer looping. This leads to an increased production of pro-inflammatory cytokines by CAFs, which in turn attract Mrc1+ myeloid cells with a pro-tumour phenotype. Accumulating myeloid cells inhibit CD8+ T-cell-mediated tumour cell killing, which results in increased tumour size in dKO animals. CAF-driven inflammatory signalling could also promote melanoma dedifferentiation through mechanisms that are yet to be determined (dashed lines). Illustration in h by Jill K. Gregory, reproduced with permission from © Mount Sinai Health System.