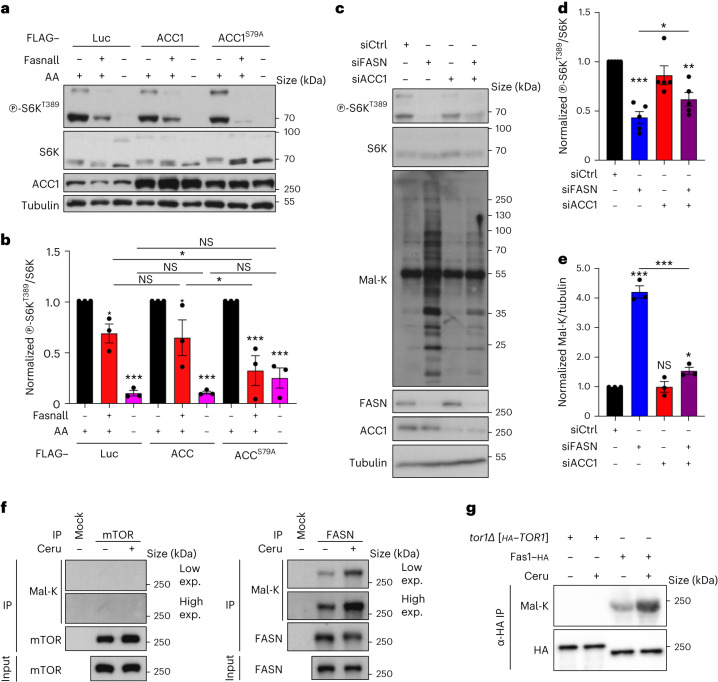
Fig. 4. Mal-CoA accumulation following perturbations to ACC1 and FASN inhibits mTORC1 independently of mTOR malonylation.
a,b, Exogenous expression of a hyperactive ACC1 mutant (ACC1S79A) cooperates with FASN inhibition to downregulate mTORC1 in HEK293FT cells without influencing the response to amino-acid (AA) starvation. The cells were transfected with vectors expressing FLAG-tagged WT ACC1, ACC1S79A or Luciferase (Luc; as the control) and treated with Fasnall (25 μM, 30 min) or AA-starvation medium (1 h) as indicated (n = 3 independent experiments). a, Phosphorylation of S6K at T389 was used to assay mTORC1 activity. b, Levels of mTORC1 activity (p-S6KT389/S6K ratio). c–e, ACC1 knockdown partially restores the increase in Mal-CoA levels and rescues the downregulation of mTORC1 caused by silencing of FASN. siCtrl, control siRNA; and siACC1, siRNA to ACC1. c, Immunoblots of HEK293T cell lysates. d, Calculated levels of mTORC1 activity (p-S6KT389/S6K ratio; n = 5 independent experiments). e, Levels of Mal-K (Mal-K/tubulin ratio; n = 3 independent experiments). f, Lack of detectable mTOR malonylation in HEK293FT cells. Endogenous mTOR (left) and FASN (right) proteins were immunoprecipitated from control (−) or cerulenin-treated (50 μM, 4 h) cells. g, Tor1 is not malonylated in yeast cells. Amino (N)-terminally HA-tagged Tor1 or C-terminally HA-tagged Fas1 was immunoprecipitated from control (−) or cerulenin-treated (10 μM, 2 h) cells cultured to the exponential phase; α-HA, anti-HA. f,g, Protein malonylation was assessed using anti-Mal-K (n = 3 independent experiments); IP, immunoprecipitate; and exp., exposure. b,d,e, Data are the mean ± s.e.m. *P < 0.05; **P < 0.005; ***P < 0.0005; and NS, not significant. Ceru, cerulenin. Source numerical data and unprocessed blots are provided.