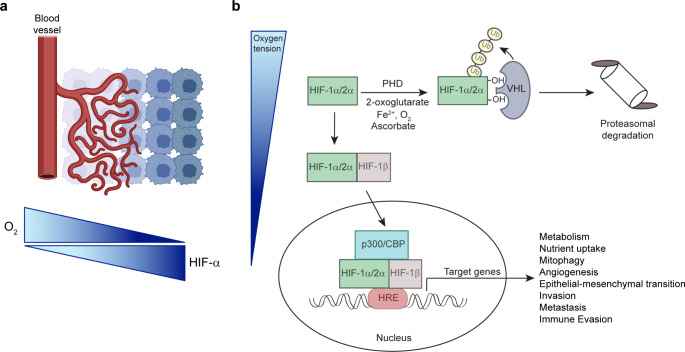
Fig. 1.
(a) In solid tumors, HIF-α is stabilized under conditions of low O2 due to reduced vascularization and the establishment of a hypoxic microenvironment. (b) Post-transcriptional regulation of HIF-α subunits. Under normoxic conditions, PHD enzymes utilize oxygen and 2-oxoglutarate as substrates to hydroxylate two proline residues in the HIF-α subunit. These hydroxylation events are required for VHL to bind, ubiquitinate, and target HIF-α for proteasomal degradation. Under hypoxia, hydroxylation is inhibited and HIF-α stabilized. HIF-α heterodimerizes with HIF-1β, interacts with the transcriptional coactivators p300 and CBP, and binds to HRE elements within regulatory regions of target genes. Illustrations were created with BioRender.