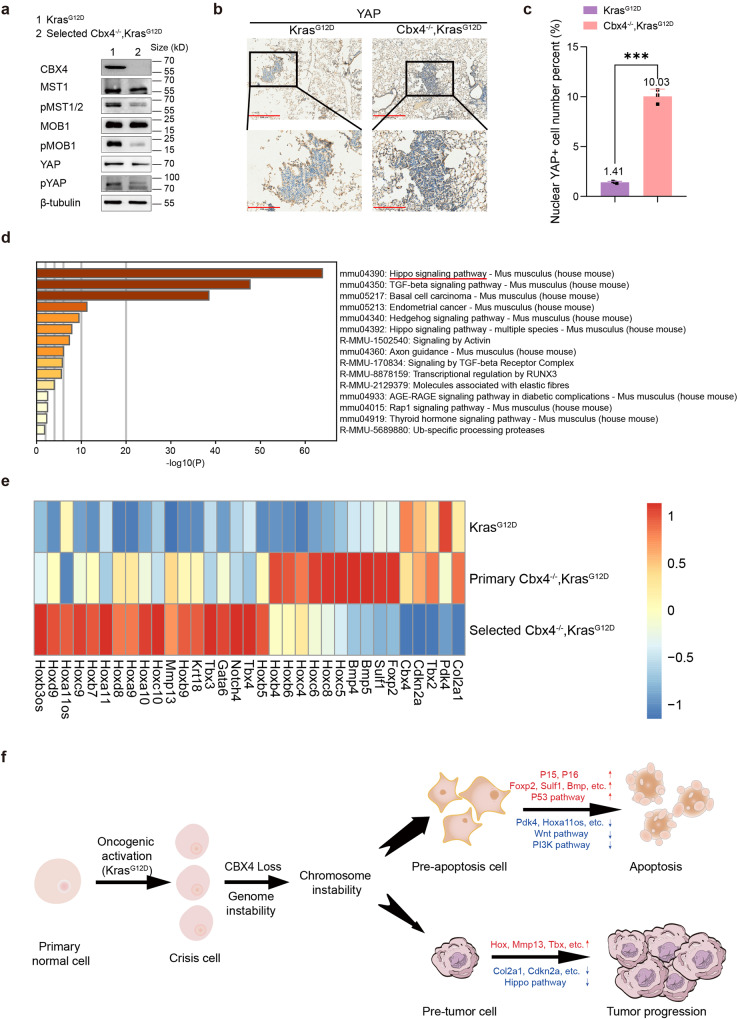
Fig. 6.
CBX4 deletion influences multiple signaling pathways including Hippo pathway and ultimately promotes KrasG12D tumorigenesis. a Western Blot of CBX4, MST1, pMST1/2, MOB1, pMOB1, YAP, pYAP and β-tubulin in KrasG12D and selected Cbx4−/−, KrasG12D MEFs. b Representative immunohistochemical staining of YAP in lung sections from KrasG12D and Cbx4−/−, KrasG12D mice. Scale bar: 500 μm (up), 200 μm (down). c Quantitative analysis of nuclear YAP positive cell percent in IHC staining of lung sections from KrasG12D and Cbx4−/−, KrasG12D mice. d GO enrichment analysis focused Hippo signaling pathway based on independent analysis. e Expression condition of several representative genes in selected Cbx4−/−, KrasG12D cells compared with KrasG12D and primary Cbx4−/−, KrasG12D groups. Upper one/row is KrasG12D, the middle one/row is primary Cbx4−/−, KrasG12D group, the lower one/row is selected Cbx4−/−, KrasG12D group. Red indicates more expression and blue means less expression. f Mechanism of CBX4 deletion in promoting tumorigenesis with KrasG12D mutation. At the early stage of cell culture, P15, P16 and other apoptosis-related genes are upregulated in the Cbx4−/−, KrasG12D cells due to chromosome instability, which lead to a large population of cell apoptosis. At the late stage of cell culture, a small population of cells survive from genomic instability acquiring stronger abilities in proliferation and transformation called “selected Cbx4−/−, KrasG12D”, due to changes of multiple pathways including inactivation of Hippo pathway, which ultimately result in tumorigenesis. Data are shown as means ± SEM. **p < 0.01, ***p < 0.001