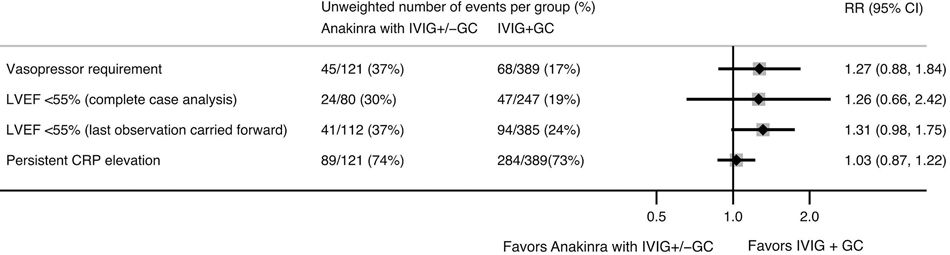
Figure 4.
Relative risks (RR) of clinical outcomes in children with MIS-C receiving anakinra as initial therapy (with intravenous immunoglobulin [IVIG] and/or glucocorticoids [GC]) compared to IVIG and GC alone, estimated using inverse probability weighted models with site-level random effects. Clinical outcomes were assessed on day 3 of treatment, except for left ventricular ejection fraction (LVEF), which was assessed on day 3–4, using either complete case analysis (N=327 LVEF recorded on day 3–4) or last observation carried forward (N=497). The direction of the average treatment effect for C-reactive protein (CRP) is represented as failure to achieve 50% reduction in CRP from admission.