Abstract
Introduction
Contemporary differences between South Asian and White ethnicities in the incidence of end-stage kidney disease (ESKD) and mortality are poorly described.
Methods
Data for all South Asian patients who had an estimated glomerular filtration rate (eGFR) measure after January 1, 2006, and 1 million randomly selected participants of other ethnicities were extracted from the Clinical Practice Research Datalink (CPRD). All participants were followed-up with from index date until ESKD, all-cause mortality, or end of study. All-cause mortality rate and ESKD incidence rate by age were described among Whites and South Asians, and adjusted hazard ratios (HRs) of these 2 outcomes by baseline eGFR estimated using Cox proportional hazard model.
Results
A total of 40,888 South Asians and 236,634 Whites were followed for a median of 5.3 and 9.4 years for ESKD incidence and mortality outcomes, respectively. All-cause mortality rates were higher among Whites than South Asians; South Asian women aged between 70 and 85 years had a slightly higher ESKD incidence rate compared to their White counterparts. Compared to Whites with a baseline eGFR of 90 ml/min per 1.73 m2, adjusted HRs for all-cause mortality were significantly lower among South Asians than Whites; however, adjusted HRs for ESKD incidence by baseline eGFR were similar in both ethnicities. Calculating South Asian eGFRs using an ethnicity-specific equation had no impact on the results.
Conclusions
South Asians experience lower mortality than Whites but not substantially higher rates of ESKD. Further research is warranted to better understand the reasons for these ethnic differences and possible impacts on chronic kidney disease (CKD) service delivery and patient outcomes.
Keywords: chronic kidney disease, ethnicity, dialysis, end-stage kidney disease, mortality, transplantation
Graphical abstract
CKD is common and places a high burden of disease on patients and high costs on healthcare systems worldwide.1 About 10% of the adult population may be affected by some degree of CKD,1 although evidence is conflicting regarding the prevalence of CKD in different ethnic groups.2
The latest population census for England and Wales recorded 9.3% of the population as being of Indian, Pakistani, or Bangladeshi ethnicity (of South Asian ethnicity).3 UK renal registry data2 consistently show that patients from minority ethnic groups are disproportionately represented among those with ESKD requiring kidney replacement therapy (KRT), either dialysis or a kidney transplant. In 2020, individuals of South Asian ethnicity represented 13.9% and 13.4% of the incident and prevalent populations receiving KRT, respectively, in the UK,2 which is much higher than the reported proportions of the general population from these ethnic minority groups. The reasons for this discrepancy are unclear.
Most patients with CKD are either undetected or managed in primary care and are not referred for specialist care at nephrology services. Early identification of CKD in the community, with appropriate treatment and monitoring of populations at risk, is therefore key to its management.
There is a perception that South Asian individuals have higher rates of CKD than individuals of White ethnicity. This was highlighted in a recent report4 as evidence of inequality in kidney care, although the evidence cited relies on data between 15- and 30-years old, and was derived from populations selected for preexisting CKD risk factors.5,6 Major changes to kidney care have taken place over this period, not least the widespread introduction of CKD staging and management guidelines.7 Clinical practice guidelines point to Asian ethnicity as a risk factor for CKD progression,7 but the evidence for this is conflicting. Some studies report faster CKD progression and increased risk of ESKD in South Asians8 whereas others report no significant differences between Whites and South Asians,9 with little overall difference in CKD incidence, prevalence, or progression between ethnicities.10 Although ethnic minority groups have higher rates of type 2 diabetes mellitus (DM) and hypertension, to date there is no definitive epidemiologic link between existing comorbidities and the higher rate of ESKD.10
We aimed to identify any ethnic differences in the incidence rate of ESKD and all-cause mortality rates in a UK based primary care cohort, and to evaluate the impact of eGFR on these outcomes by sex and ethnicity, accounting for existing cardiovascular comorbidities.
Methods
Study Design and Data Source
In this descriptive, retrospective, longitudinal cohort study, patient-level data were extracted from the CPRD, which contains healthcare information from over 35 million patients registered with over 1400 primary care practices in the UK (https://www.cprd.com/). In previous studies, CPRD has been shown to be representative of the UK population in terms of age, sex, ethnicity, and socioeconomic status.11,12 Linkage to hospital data collected in hospitals as Hospital Episodes Statistics Admitted Patient Care and the Office of National Statistics Death Registration provided information on hospital episodes and mortality as well as validation of ethnicity codes. The study protocol was approved by the CPRD Independent Scientific Advisory Committee (ISAC No. 18_158R). We conducted and reported this study following the RECORD (REporting of studies Conducted using Observational Routinely-collected Data (RECORD) checklist.13
Study Population
Data for all patients coded as South Asian in CPRD were extracted as the exposed group and an additional 1 million participants with other coded ethnicities were randomly selected as the non-exposed group. Ethnicity was self-reported when the patient registered at the practice, and the use of ethnicity data which is well recorded from 2006 onward in CPRD, can maximize completeness and comparability with the UK population.14 Participants with no linkage to Hospital Episodes Statistics or the Office of National Statistics, with conflict coding of ethnicity in Hospital Episodes Statistics and CPRD, or without a serum creatinine measure after January 1, 2006 were excluded. The index date was the first serum creatinine measure date after January 1, 2006. Participants with a code for renal dialysis, kidney transplant or eGFR (based on the Chronic Kidney Disease-Epidemiology Collaboration [CKD-EPI] equation15) lower than 15 ml/min per 1.73 m2 before or at the index date, or age of <18 years before or on the index date, were excluded. In addition, all participants were required to have at least 12 months’ registration in an “up-to-standard” practice before the index date. Only individuals of White ethnicity constituted the nonexposed group and individuals identifying as mixed race or ethnicity were excluded.
Covariates and Outcomes
Sociodemographic factors (age at index date and sex) were extracted from CPRD. Index of multiple deprivation 2010 was linked at practice-level to derive information on socioeconomic status. Body mass index, smoking status, diastolic and systolic blood pressure, and total cholesterol were extracted from CPRD using the closest value to the index date. We determined the history of myocardial infarction, ischemic heart disease, stroke, hypertension, heart failure, cancer, dementia, and DM, if the patient had a diagnosis code in CPRD or Hospital Episodes Statistics any time before the index date. We also counted the number of previous hospital admissions before the index date to quantify health care utilization.
All participants were followed-up from the index date until the occurrence of the outcomes of interest, namely ESKD and all-cause mortality, end of the study (transfer-out CPRD practice date, last data collection date, or December 31, 2017, for ESKD events; February 14, 2018, for mortality outcomes), or death, whichever occurred first. ESKD events included the initiation of dialysis, renal transplantation, and eGFR decline to <15 ml/min per 1.73m2, whichever occurred first.
Statistical Analysis
Complete-case baseline characteristics (i.e., at index date) were compared between White and South Asians. Numbers and percentages were calculated for categorical variables; median and interquartile range (IQR) were presented for continuous variables. Comparisons between the 2 ethnic groups were conducted using Pearson’s χ2 test and Wilcoxon rank-sum test for categorical and continuous variables, respectively.
We presented the crude cumulative incidence of ESKD and all-cause death by follow-up time among Whites and South Asians, where the competing risk of death was accounted for in the incidence of ESKD. We used the “stcompet” command in Stata16 to generate nonparametric cumulative incidence in the presence of competing events. We then split the risk-time in 1-year intervals by attained age and used Poisson regressions with an interaction term between the spline transformed age and ethnicity to estimate age-specific all-cause mortality and ESKD incidence rates with 95% confidence intervals by age, sex, and ethnicity. We then calculated the age-specific and sex-specific unadjusted rate ratios (comparing South Asians to Whites) for all-cause mortality and ESKD incidence.
After checking the plots of the scaled Schoenfeld residuals, we used Cox proportional hazard models to estimate the HRs for ESKD and all-cause mortality across the range of baseline eGFR in men and women; to account for nonlinear associations, models included an interaction term between ethnicity and a restricted cubic spline transformation (with 4 knots) of baseline eGFR and were adjusted for age at baseline, index of multiple deprivation, smoking status, systolic and diastolic blood pressure, body mass index, total cholesterol, the number of previous admissions, myocardial infarction, ischemic heart disease, stroke, heart failure, DM, cancer, and dementia. People of White ethnicity with an eGFR of 90 ml/min per 1.73 m2 were used as the reference to calculate the HRs for different values of eGFR in both White and South Asian people. To account for the impact of missing data, we conducted multiple imputation analysis with 10 times and combined the HRs with Rubin’s Rules as sensitivity analyses.17
For exploratory analysis, we repeated the above analysis using eGFR calculated with the CKD-EPI PK equation, which has been developed in South Asian Pakistani population and has been shown to have an improved accuracy than the CKD-EPI equation to estimate GFR in South Asian individuals.18
All statistical analyses were conducted in R for Window 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) using the “Epi” and “rms” packages and graphs were plotted in Stata/IC version 16.0. Clinical codes for the definition of the population, outcomes and covariates and tabular results are reported at https://github.com/supingling/SA_CKD.
Results
Cohort Characteristics
Overall, 277,522 individuals (156,187 [56.3%] female) were included in the analysis; the patient flowchart is shown in Figure 1. There were 40,888 South Asian and 236,634 White individuals, who were followed-up with for a median of 5.3 years (IQR: 2.6–8.1) and 9.4 years (6.3–11.2) for incidence of ESKD and mortality outcomes, respectively. Baseline characteristics are reported in Table 1. Missing data patterns are reported in Supplementary Table S1. The median age of the overall population was 53.9 years (40.0–67.7), with individuals of South Asian ethnicity being younger (median [IQR]: 38.7 years [30.6–50.8]) than those of White ethnicity (56.8 years [43.0–69.5]). There were more South Asians from the most deprived areas than Whites (29.9% vs. 24.4%). The median baseline eGFR was 83 ml/min per 1.73 m2 (IQR: 68–99) in the overall population; South Asians had a higher baseline eGFR (median [IQR]: 100 ml/min per 1.73 m2 (85–114)] than Whites (81 ml/min per 1.73 m2 [66–95]). When eGFR was calculated by CKD-EPI, more South Asians were classified into higher eGFR strata compared to Whites. Estimation of eGFR by CKD-EPI-PK placed more South Asians in lower eGFR strata, bringing the relative proportions closer to those of Whites (Table 1).
Figure 1.

Flowchart of participants’ selection. CPRD, Clinical Practice Research Datalink; ESRD, End stage renal disease; HES, Hospital Episodes Statistics; ONS, Office for National Statistics; SA, South Asian; SC, Serum creatinine.
Table 1.
Baseline characteristics and outcomes by ethnicity
| Characteristics | White | South Asian |
|---|---|---|
| Population (N) | 236,634 | 40,888 |
| Age (yrs) | 56.8 (43.0–69.5) | 38.7 (30.6–50.9) |
| Sex | ||
| Male | 103,088 (43.6%) | 18,247 (44.6%) |
| Female | 133,546 (56.4%) | 22,641 (55.4%) |
| Index of multiple deprivation, quintile | ||
| 1–least deprived | 38348 (16.2%) | 5237 (12.8%) |
| 2 | 44748 (18.9%) | 6235 (15.2%) |
| 3 | 45995 (19.4%) | 8273 (20.2%) |
| 4 | 49892 (21.1%) | 8912 (21.8%) |
| 5–most deprived | 57651 (24.4%) | 12231 (29.9%) |
| eGFR (CKD-EPI)a | 81 (66–95) | 100 (85–114) |
| CKD-EPI eGFRa strata: | ||
| >90 | 78,968 (33.4%) | 27,391 (67.0%) |
| 60–89 | 117,722 (49.7%) | 11,885 (29.1%) |
| 45–59 | 28,509 (12.0%) | 1239 (3.0%) |
| 30–44 | 9551 (4.0%) | 312 (0.8%) |
| 15–29 | 1884 (0.8%) | 61 (0.1%) |
| eGFR (CKD-EPI-PK)a | - | 90 (76–103) |
| CKD-EPI-PK eGFRa strata: | ||
| >90 | - | 20,369 (49.8%) |
| 60–89 | - | 17,237 (42.2%) |
| 45–59 | - | 2510 (6.1%) |
| 30–44 | - | 649 (1.6%) |
| 15–29 | - | 116 (0.3%) |
| Last HbA1C (%) | 5.8 (5.4–6.5) | 5.9 (5.5–6.7) |
| Body mass index (kg/m2) | 26 (23–30) | 25 (22–28) |
| Diastolic blood pressure (mm Hg) | 80 (70–86) | 77 (70–84) |
| Systolic blood pressure (mm Hg) | 132 (120–145) | 122 (110–134) |
| Total cholesterol (mmol/l) | 5.2 (4.5–6.0) | 4.9 (4.2–5.6) |
| Smoking status | ||
| Yes | 47,895 (20.2%) | 4693 (11.5%) |
| No | 121,811 (51.5%) | 32,747 (80.1%) |
| Ex-smoker | 65,832 (27.8%) | 3201 (7.8%) |
| Comorbidities | ||
| Myocardial infarction | 8334 (3.5%) | 723 (1.8%) |
| Ischemic heart disease | 22,368 (9.5%) | 1891 (4.6%) |
| Stroke | 10,977 (4.6%) | 542 (1.3%) |
| Heart failure | 5431 (2.3%) | 309 (0.8%) |
| Diabetes | 19,067 (8.1%) | 4987 (12.2%) |
| Cancer | 18,787 (7.9%) | 613 (1.5%) |
| Dementia | 2268 (1.0%) | 106 (0.3%) |
| Number of previous admissions | 1 (0–3) | 1 (0–2) |
CKD, chronic kidney disease; CKD-EPI-PK, CKD-Epidemiology Collaboration-Pakistan; ESKD, end-stage kidney disease; eGFR, estimated glomerular filtration rate.
Numbers are reported as frequency (%) or median (interquartile range).
eGFR in ml/min per 1.73 m2.
Consistent with the age difference, all major cardiovascular comorbidities were more common in Whites than South Asians, except DM, which was more common in South Asians. Compared to Whites, South Asians were more likely to be nonsmokers, have a lower body mass index, lower blood pressure and lower total cholesterol, but higher HbA1C at baseline (Table 1).
Rates of Mortality and ESKD by Age, Sex, and Ethnicity
During 2,038,107 and 303,659 person-years’ follow-up, 46,757 (19.8%) and 1393 (3.4%) deaths occurred among Whites and South Asians, respectively (Table 2). The number of ESKD events was small for both ethnicities; there were 5201 (2.2%) events during 1,338,950 person-years in Whites, and 319 (0.8%) during 187,995 person-years in South Asians (Table 2). The composition of ESKD by ethnicity is shown in Supplementary Table S2, and there was no noticeable difference between Whites and South Asians. The corresponding cumulative incidence of ESKD and all-cause deaths, accounting for competing risk, are shown in Supplementary Figure S1. Both White and South Asian individuals displayed increasing all-cause mortality by age. However, in both men and women at all ages, all-cause mortality rates were significantly higher among Whites compared to South Asians at all ages (Figure 2, Supplementary Table S3).
Table 2.
The number of events, person-years and overall crude rates among Whites and South Asians
| Event | Whites (N = 236,634) |
South Asians (N = 40,888) |
||||
|---|---|---|---|---|---|---|
| No. of events | Person-yrs | Crude rates (per 1000 person-yrs) | No. of events | Person-yrs | Crude rates (per 1000 person-yrs) | |
| All-cause deaths | 46,757 (19.8%) | 2,038,107 | 22.9 (22.7, 23.2) | 1393 (3.4%) | 303,659 | 4.6 (4.4, 4.8) |
| ESKD | 5201 (2.2%) | 1,338,950 | 3.9 (3.9, 4.0) | 319 (0.8%) | 187,995 | 1.7 (1.5, 1.9) |
ESKD, end-stage kidney disease
Rates are shown with a 95% confidence interval.
Figure 2.
All-cause mortality rate and unadjusted rate ratio by age among women and men. Top panel: All-cause mortality rate (per 1000 person-years) among Whites and South Asians. Bottom panel: Unadjusted All-cause mortality rate ratio comparing South Asian to White (reference).
Compared to White men, in South Asian men there was a minimally increased ESKD incidence around the age of 50 years (Figure 3, Supplementary Table S3). In South Asian women there was a larger, significant increase in the incidence of ESKD between 70 and 82 years of age compared to White women. For example, at age 75 years the rate of ESKD in White women was 54.1 per 10,000 person years (95% confidence interval: 50.4–58.1) compared to a rate of 81.4 (61.4–107.1) in South Asian women (Figure 3, Supplementary Table S3).
Figure 3.
End-stage kidney disease incidence rate and unadjusted rate ratio by age among women and men. Top panel: End-stage kidney disease incidence rate (per 1000 person-years) among Whites and South Asians. Bottom panel: Unadjusted end-stage kidney disease incidence rate ratio comparing South Asians to Whites (reference).
Hazard Ratios of ESKD and All-Cause Death, by eGFR and Ethnicity
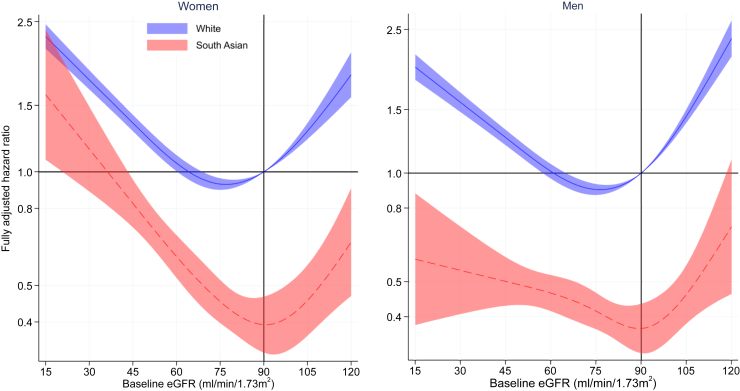
Among both Whites and South Asians, the relationship between adjusted HRs for all-cause mortality and baseline eGFR is represented by a U-shaped curve, such that the HR for mortality is increased at both lower and higher levels of eGFR (Figure 4). Using an eGFR at 90 ml/min per 1.73 m2 as the reference for mortality among Whites (i.e., HR = 1), the adjusted HRs for all-cause mortality were significantly lower in both male and female South Asians compared to Whites at most levels of CKD-EPI eGFR, except at eGFR levels less than 25 ml/min per 1.73m2 in females where the curves for each ethnicity overlap.
Figure 4.
Fully adjusted hazard ratio of eGFR for all-cause mortality by ethnicity. Models included an interaction between eGFR and ethnicity and adjusted for age at baseline, index of multiple deprivation, smoking status, systolic and diastolic blood pressure, body mass index, total cholesterol, number of previous admissions, myocardial infarction, ischemic heart disease, stroke, heart failure, diabetes, cancer, and dementia. The reference is White with an eGFR of 90 ml/min per 1.73 m2. eGFR, estimated glomerular filtration rate
Predictably, the risk of ESKD was inversely related to the level of baseline CKD-EPI eGFR in both South Asians and White subjects. However, the adjusted HRs for ESKD by baseline CKD-EPI eGFR showed no evidence of a difference between South Asians and Whites, except in South Asian females who had lower HR for ESKD compared to White females with a baseline eGFR around 90 ml/min per 1.73 m2 (Figure 5).
Figure 5.
Fully adjusted hazard ratio of eGFR for end-stage kidney disease incidence by ethnicity. Models included an interaction between eGFR and ethnicity and adjusted for age at baseline, index of multiple deprivation, smoking status, systolic and diastolic blood pressure, body mass index, total cholesterol, number of previous admissions, myocardial infarction, ischemic heart disease, stroke, heart failure, diabetes, cancer, and dementia. The reference is white with an eGFR of 90 ml/min per 1.73 m2. eGFR, estimated glomerular filtration rate.
Multiple imputation analyses for adjusted HRs for all-cause mortality and ESKD are shown in Supplementary Figures S2 and S3, and Supplementary Table S4, where the estimates were very similar to those of main analyses.
Effects of eGFR Calculated Using the CKD-EPI-PK Equation in South Asians
Calculating eGFR from serum creatinine using the CKD-EPI-PK equation resulted in an apparent reduction in South Asian eGFR of about 10% compared to the CKD-EPI eGFR (Table 1). The small differences in unadjusted ESKD rates by age were somewhat accentuated in women when South Asian eGFR was calculated by CKD-EPI-PK (Supplementary Figure S4). However, HR curves for all-cause mortality by eGFR were consistent with those created using CKD-EPI eGFR (Supplementary Figure S5). Conversely, when the CKD-EPI-PK was used to calculate eGFR, the HR for ESKD by eGFR was significantly lower in South Asian males compared to White males (Supplementary Figure S6).
Discussion
To our knowledge, this is the largest analysis of the pattern of CKD risk among South Asian individuals compared to White individuals. Herein, we describe ethnic differences in mortality and ESKD incidence in a large contemporary UK adult primary care population. Our data show that, compared to Whites, South Asians have lower rates of mortality across age and eGFR, but an increased risk of ESKD in some age groups of men and women. In addition, when adjusted for multiple cardiovascular and other risk factors, South Asian men and women were at significantly lower relative risk of mortality for all CKD-EPI eGFRs, except for the very lowest eGFR levels in South Asian women.
South Asian individuals reached ESKD less commonly than Whites, and whereas the risk of future ESKD at any baseline eGFR was broadly similar for both South Asian and White individuals, South Asian patients were younger than Whites when they commenced KRT. Registry data also show that South Asians incident to KRT are younger than Whites and are most highly represented as a proportion of the 35 to 44 years age group.19 Conversely, Whites are most highly represented as a proportion of patients over 75 years of age in the incident KRT population. For prevalent KRT patients however, the highest proportions of both Whites and South Asians are in the 55 to 64 years age group.19 In 2018, South Asian patients in the UK commenced KRT (dialysis or transplantation) with a mean eGFR of 6.7 ml/min per 1.73 m2 compared to 7.6 ml/min per 1.73 m2 for Whites (personal communication, Prof James Medcalf, Medical Director, UK Renal Registry) and therefore the clinical threshold for commencing both South Asian and White ethnicities on KRT appears similar. Taken together therefore, the registry and the current data suggest that South Asians reach ESKD earlier than Whites and then survive for longer.
Although it is well-described that South Asians experience higher rates of morbidity and mortality because of ischemic heart disease and other circulatory disorders than Whites,20,21 in the current study significantly fewer South Asians had high blood pressure and other cardiovascular diseases, despite higher rates of diabetes. The observation that the South Asian cohort were significantly younger than the Whites is the probable explanation for this finding. Our mortality findings are consistent with the lower age standardized mortality rates previously described among Bangladeshi, Indian, and Pakistani groups compared to White ethnicities in England and Wales.22,23
The development and progression of CKD in South Asian people is not well-described, but understanding this disease trajectory is important for planning of renal services, particularly in areas with high proportions of ethnic minority populations. Individuals of South Asian ethnicity in the UK are overrepresented in both incident and prevalent populations of patients receiving renal replacement therapy by about 2-fold compared to White patients,19 based on the breakdown of minority ethnic population percentages recorded in the most recent UK census data.3 These observations can only be explained by either proportionally more South Asian individuals reaching ESKD and starting KRT and/or fewer South Asians than Whites discontinuing KRT, or potentially inaccuracies in census data.
Although the current cohort was not selected as a “CKD cohort,” that the participants had their renal function measured indicates a measure of kidney concern on the part of their health care providers. The current data indicate that South Asians have their renal function measured at a younger age than Whites. This clinical behavior is consistent with the higher prevalence of DM in the South Asian community and driven by the existence of financial incentives for blood testing in patients with, or at risk of DM. This is also consistent with our present observation that South Asian patients commence KRT at an earlier age than White patients.
Despite having less cardiovascular disease, South Asian women and men display an increased incidence of ESKD in some age groups. This observation may also be related to the higher proportion of South Asians with diabetes. The currently observed ethnic differences in ESKD incidence are modest however, and whether this is sufficient to explain the robustly documented epidemiology of incident South Asian patients beginning KRT is uncertain. However, both the mortality rates and adjusted HRs for South Asians are lower at all ages and eGFRs, suggesting that more South Asians with CKD may survive to the point of needing KRT and may then survive longer when receiving KRT, thus accounting for the greater numbers of South Asian patients on KRT reported by the UK Renal Registry.19 In Canada, South Asian patients experience lower mortality than White patients for unclear reasons.24
The current findings differ in some respects from those of previous work8,25 comparing South Asian and White outcomes in smaller and older cohorts selected for the presence of CKD. In the earlier analyses of CKD cohorts, South Asian patients also had a lower risk of death but more rapid progression of CKD and a higher risk of ESKD compared to Whites. The discrepancy in ESKD risk between these reports and the current data may be explained by the higher baseline CKD-EPI eGFR in the current South Asian cohort of 99 ml/min per 1.73 m2 compared to CKD-EPI eGFR 27 to 47 ml/min per 1.73 m2 in the earlier work. In addition, the definitions of ESKD vary in the other papers compared to the present analysis. In the current paper, ESKD was defined also as an eGFR <15 ml/min per 1.73 m2 whereas in earlier studies it was defined as the commencement of renal replacement therapy. We believe that our approach mitigates against KRT coding inaccuracies in primary care records.
Eastwood et al.26 studied longitudinal relationships between markers of CKD and mortality and morbidity in a relatively small cohort of Europeans and South Asians in London, randomly selected from general practices. They found lower mortality rates in South Asians, a weaker relationship between reduced eGFR and mortality in South Asians than in Europeans, but a stronger relationship between urine albumin-to-creatinine ratio and adverse outcomes in South Asians. This finding differs from the current study where we observed a clear association between increasing mortality and decreasing eGFR in both White and South Asian individuals, although whereas this relationship was qualitatively similar between both groups, South Asians experience lower mortality risk at virtually all levels of eGFR.
Recently, there have been calls to move away from corrections to eGFR-calculating equations based on race.27 However, there is also longstanding concern that current creatinine based eGFR equations overestimate eGFR in South Asian people28,29 but a recent National Institute for Health and Care Excellence evidence synthesis concluded that for a variety of reasons, based on current evidence, it was not possible to make specific recommendations for eGFR adjustments for South Asians in the UK.7 Weaker associations between eGFR and outcomes in South Asians may be due to poor validity of CKD-EPI eGFR as a surrogate for measured GFR in South Asians. To attempt to address this, we recalculated all South Asian eGFRs using the CKD-EPI-PK equation, developed in a South Asian population in Pakistan, where it enhances the performance of the CKD-EPI equation by reducing the overestimating effect of CKD-EPI.18 In the current analysis, whereas South Asian CKD-EPI-PK calculated eGFRs were appreciably lower than those calculated by CKD-EPI, the overall relationship of eGFR to outcomes was unchanged.
The strengths of this study include the large sample size of individuals accessing a standardized healthcare system. The sample is representative of the general population and is not a referred CKD cohort of individuals. The use of routinely collected data is prone to coding error, however we choose outcomes which are proven to be reliable in the CPRD dataset, including mortality and eGFR.30,31 In addition, we have accounted for nonlinear associations of eGFR in relation to ESKD incidence and mortality; and by including patients who progressed to an eGFR <15 ml/min per 1.73 m2, we are confident we have captured patients with CKD progression to ESKD over the course of the study. However, this definition of ESKD hampers the comparisons with registry data where commencement of KRT is reported. The absence of albumin-to-creatinine ratio or other proteinuria data is a weakness because proteinuria is a strong predictor of future CKD decline in South Asian people.26 We also lacked lifestyle data such as physical activity levels, which have been shown to be associated with CKD,32 and cannot definitively exclude residual confounding (the risk of developing ESRD could be related to some characteristics which differ between ethnic groups but have not been accounted for) or exposure ascertainment bias (the probability of having eGFR measured is higher in a person at higher risk of developing ESRD; however, in the UK primary care, eGFR is frequently measured).
Taken together, our results suggest that South Asians do not experience a substantially higher rate of ESKD than the White population. Because previous studies and registry data suggest that South Asians may survive longer than Whites when receiving KRT,5,19,33 further investigations are required to explore the individual characteristics and the risk factors influencing ethnic-specific kidney disease trajectories leading to ESKD or KRT and renal mortality.
Disclosure
KK reports grants and personal fees from AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim; personal fees from Bayer, Abbott, Amgen, Napp; and grants from Oramed Pharmaceuticals, Applied Therapeutics, outside the submitted work. All other authors report nothing to disclose.
Acknowledgments
Funding
Funding for the study was provided by the National Institute for Health and Care Research Applied Research Centre East Midlands.
Data Sharing
Data access is via permission from CPRD only; please send any enquiries to enquiries@cprd.com. Clinical codes for the definition of the population, outcomes and covariates, and tabular results are reported at https://github.com/supingling/SA_CKD.
Author Contributions
GX, FZ, and NJB designed the study. GX and FZ defined clinical codes. SL cleaned and analyzed the data. FZ supported data analysis. SL, GX, and NJB drafted the article. All authors contributed to the interpretation of the data, critically revised the article, and approved the final version. SL and FZ have full access to all the data. NJB is responsible for the integrity of the work as a whole.
Footnotes
Figure S1. Cumulative incidence of ESKD and all-cause death among Whites and South Asians by follow-up time.
Figure S2. Fully adjusted hazard ratio of eGFR for all-cause mortality by ethnicity (multiple imputation).
Figure S3. Fully adjusted hazard ratio of eGFR for end-stage kidney disease incidence by ethnicity (multiple imputation).
Figure S4. End-stage kidney disease incidence rate and unadjusted rate ratio by age in women and men.
Figure S5. Fully adjusted hazard ratio for all-cause mortality by ethnicity (CKD-EPI-PK for South Asians).
Figure S6. Fully adjusted hazard ratio for end-stage kidney disease incidence by ethnicity (CKD-EPI-PK for South Asians).
Table S1. Missing data pattern.
Table S2. The composition of end-stage kidney disease outcome.
Table S3. All-cause mortality rate, end-stage kidney disease incidence rate, and their rate ratio comparing White to South Asian by age.
Table S4. Adjusted hazard ratios of eGFR for all-cause mortality and end-stage kidney disease incidence.
RECORD (REporting of studies Conducted using Observational Routinely-collected Data (RECORD) checklist.
Supplementary Material
Figure S1. Cumulative incidence of ESKD and all-cause death among Whites and South Asians by follow-up time
Figure S2. Fully adjusted hazard ratio of eGFR for all-cause mortality by ethnicity (multiple imputation)
Figure S3. Fully adjusted hazard ratio of eGFR for end-stage kidney disease incidence by ethnicity (multiple imputation)
Figure S4. End-stage kidney disease incidence rate and unadjusted rate ratio by age in women and men
Figure S5. Fully adjusted hazard ratio for all-cause mortality by ethnicity (CKD-EPI-PK for South Asians)
Figure S6. Fully adjusted hazard ratio for end-stage kidney disease incidence by ethnicity (CKD-EPI-PK for South Asians)
Table S1. Missing data pattern
Table S2. The composition of end-stage kidney disease outcome
Table S3. All-cause mortality rate, end-stage kidney disease incidence rate, and their rate ratio comparing White to South Asian by age
Table S4. Adjusted hazard ratios of eGFR for all-cause mortality and end-stage kidney disease incidence
RECORD (REporting of studies Conducted using Observational Routinely-collected Data (RECORD) checklist.
References
- 1.Sundström J., Bodegard J., Bollmann A., et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2.4 million patients from 11 countries: the CaReMe CKD study. Lancet Reg Health Eur. 2022 doi: 10.1016/j.lanepe.2022.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill N.R., Fatoba S.T., Oke J.L., et al. Global prevalence of chronic kidney disease-a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office for National Statistics (ONS) Ethnic Group, England and Wales: census. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/bulletins/ethnicgroupenglandandwales/census2021
- 4.Kidney Research UK Kidney health inequalities in the UK–an agenda for change. https://www.kidneyresearchuk.org/wp-content/uploads/2019/09/Health_Inequalities_lay_report_FINAL_WEB_20190311.pdf
- 5.Roderick P., Byrne C., Casula A., et al. Survival of patients from South Asian and Black populations starting renal replacement therapy in England and Wales. Nephrol Dial Transplant. 2009;24:3774–3782. doi: 10.1093/ndt/gfp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hull S., Dreyer G., Badrick E., Chesser A., Yaqoob M.M. The relationship of ethnicity to the prevalence and management of hypertension and associated chronic kidney disease. BMC Nephrol. 2011;12:41. doi: 10.1186/1471-2369-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and care Excellence Chronic kidney disease: assessment and management. https://www.nice.org.uk/guidance/ng203 [PubMed]
- 8.Barbour S.J., Er L., Djurdjev O., Karim M., Levin A. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant. 2010;25:3663–3672. doi: 10.1093/ndt/gfq189. [DOI] [PubMed] [Google Scholar]
- 9.Mathur R., Dreyer G., Yaqoob M.M., Hull S.A. Ethnic differences in the progression of chronic kidney disease and risk of death in a UK diabetic population: an observational cohort study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen C.P., Matsushita K., Coresh J., et al. Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney Int. 2014;86:819–827. doi: 10.1038/ki.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahadevan P., Harley M., Fordyce S., et al. Completeness and representativeness of small area socioeconomic data linked with the UK Clinical Practice Research Datalink (CPRD) J Epidemiol Community Health. 2022;76:880–886. doi: 10.1136/jech-2022-219200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrett E., Gallagher A.M., Bhaskaran K., et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benchimol E.I., Smeeth L., Guttmann A., et al. The REporting of studies Conducted using Observational Routinely collected health Data (RECORD) Statement. PLOS Med. 2015;12 doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur R., Bhaskaran K., Chaturvedi N., et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2014;36:684–692. doi: 10.1093/pubmed/fdt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EconPapers. STCOMPET: Stata module to generate cumulative incidence in presence of competing events. https://EconPapers.repec.org/RePEc:boc:bocode:s431301 Accessed May 12, 2023.
- 17.Rubin D.B. Vol. 81. John Wiley & Sons; 2004. (Multiple Imputation for Nonresponse in Surveys). [Google Scholar]
- 18.Jessani S., Levey A.S., Bux R., et al. Estimation of GFR in South Asians: a study from the general population in Pakistan. Am J Kidney Dis. 2014;63:49–58. doi: 10.1053/j.ajkd.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Kidney Association UK renal registry 24th annual report-data to 31/12/2020. https://ukkidney.org/audit-research/annual-report
- 20.Chaturvedi N. Ethnic differences in cardiovascular disease. Heart. 2003;89:681–686. doi: 10.1136/heart.89.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wild S.H., Fischbacher C., Brock A., Griffiths C., Bhopal R. Mortality from all causes and circulatory disease by country of birth in England and Wales 2001–2003. J Public Health (Oxf) 2007;29:191–198. doi: 10.1093/pubmed/fdm010. [DOI] [PubMed] [Google Scholar]
- 22.Office for National Statistics (ONS) Ethnic differences in life expectancy and mortality from selected causes in England and Wales: 2011 to 2014. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/articles/ethnicdifferencesinlifeexpectancyandmortalityfromselectedcausesinenglandandwales/2011to2014#:∼:text=In%20the%20period%202011%20to,life%20expectancy%20than%20most%20groups
- 23.Office for National Statistics (ONS) Age standardised mortality rates for England and Wales by sex and ethnic group. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/agestandardisedmortalityratesforenglandandwalesbysexandethnicgroup
- 24.Robinson B.M., Akizawa T., Jager K.J., Kerr P.G., Saran R., Pisoni R.L. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/s0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Major R.W., Shepherd D., Medcalf J.F., Xu G., Gray L.J., Brunskill N.J. Comorbidities and outcomes in South Asian individuals with chronic kidney disease: an observational primary care cohort. Nephrol Dial Transplant. 2021;37:108–114. doi: 10.1093/ndt/gfaa291. [DOI] [PubMed] [Google Scholar]
- 26.Eastwood S.V., Chaturvedi N., Sattar N., Welsh P.I., Hughes A.D., Tillin T. Impact of kidney function on cardiovascular risk and mortality: a comparison of South Asian and European cohorts. Am J Nephrol. 2019;50:425–433. doi: 10.1159/000503873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delgado C., Baweja M., Crews D.C., et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79:268–288.e1. doi: 10.1053/j.ajkd.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Modi G.K., Jha V. Disparate chronic kidney disease-associated outcomes in South Asians ethnicity or estimated glomerular filtration rate? Am J Nephrol. 2019;50:422–424. doi: 10.1159/000503874. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V., Yadav A.K., Yasuda Y., et al. Existing creatinine-based equations overestimate glomerular filtration rate in Indians. BMC Nephrol. 2018;19:22. doi: 10.1186/s12882-018-0813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher A.M., Dedman D., Padmanabhan S., Leufkens H.G.M., de Vries F. The accuracy of date of death recording in the Clinical Practice Research Datalink gold database in England compared with the Office for National Statistics death registrations. Pharmacoepidemiol Drug Saf. 2019;28:563–569. doi: 10.1002/pds.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maru S., Koch G.G., Stender M., et al. Antidiabetic drugs and heart failure risk in patients with type 2 diabetes in the U.K. primary care setting. Diabetes Care. 2005;28:20–26. doi: 10.2337/diacare.28.1.20. [DOI] [PubMed] [Google Scholar]
- 32.Bharakhada N., Yates T., Davies M.J., et al. Association of sitting time and physical activity with CKD: a cross-sectional study in family practices. Am J Kidney Dis. 2012;60:583–590. doi: 10.1053/j.ajkd.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Pei Y.P., Greenwood C.M., Chery A.L., Wu G.G. Racial differences in survival of patients on dialysis. Kidney Int. 2000;58:1293–1299. doi: 10.1046/j.1523-1755.2000.00285.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.