Figure 3.
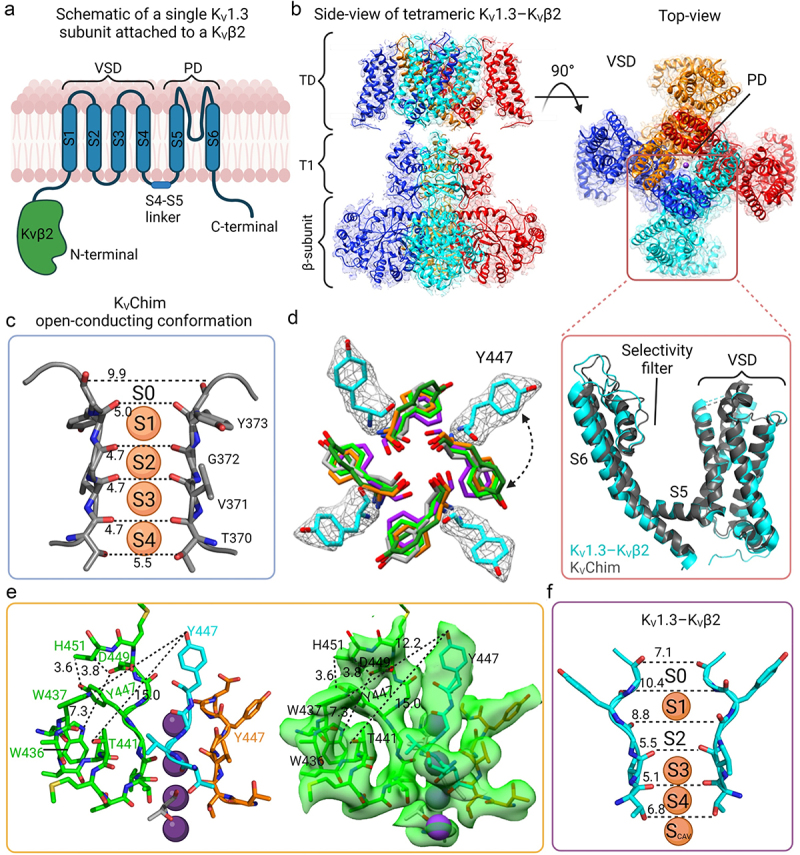
Structure of KV1.3-KVβ2 in non-conducting, C-type inactivated conformation. a) Schematic view of a single subunit of KV1.3 with the transmembrane region in blue and the N-terminal intracellular β-subunit in green. b) The cryo-EM density map of KV1.3-KVβ2 side-view (left) and top view (right). The inset shows a superimposition of the VSDs and selectivity filters of KV1.3-KVβ2 (cyan) (PDB 7WF3) and KVChim (gray) (PDB 2R9R). c) Selectivity filter of KVChim (PDB 2R9R) showing the distances between the carbonyl O atoms of two subunits and the four K+ in the selectivity filter at S1, S2, S3, S4 positions. d) Overlay of the KV1.3-KVβ2 Tyr447 (cyan) and equivalent aromatic residues in the selectivity filter of KVChim Tyr373 (gray), hERG F627 (orange) (PDB 5VA1), Eag-1 Phe439 (purple) (PDB 5K7L) and KcsA Tyr78 (neon green) (PDB 1K4C). The cryo-EM density of KV1.3-KVβ2 Tyr447 in white mesh is overlapped with the model represented as stick cyan. e) the four subunits of KV1.3-KVβ2 (green, cyan, orange and gray) with the new position of Tyr447 and D449 being stabilized by intra-subunit hydrogen bond interactions with His451, a residue at the external entrance to the KV1.3 pore. The residues of each subunit are represented as sticks. On the right, the cryo-EM density is represented as a transparent green surface. f) Selectivity filter of KV1.3-KVβ2 showing the difference in the position of the aromatic Tyr447 residue in the selectivity filter compared to KVChim in (c). The widened outer selectivity filter of KV1.3-KVβ2 shows K+ at ion-binding sites S1, S3, S4 but S2 is empty.
