Figure 5.
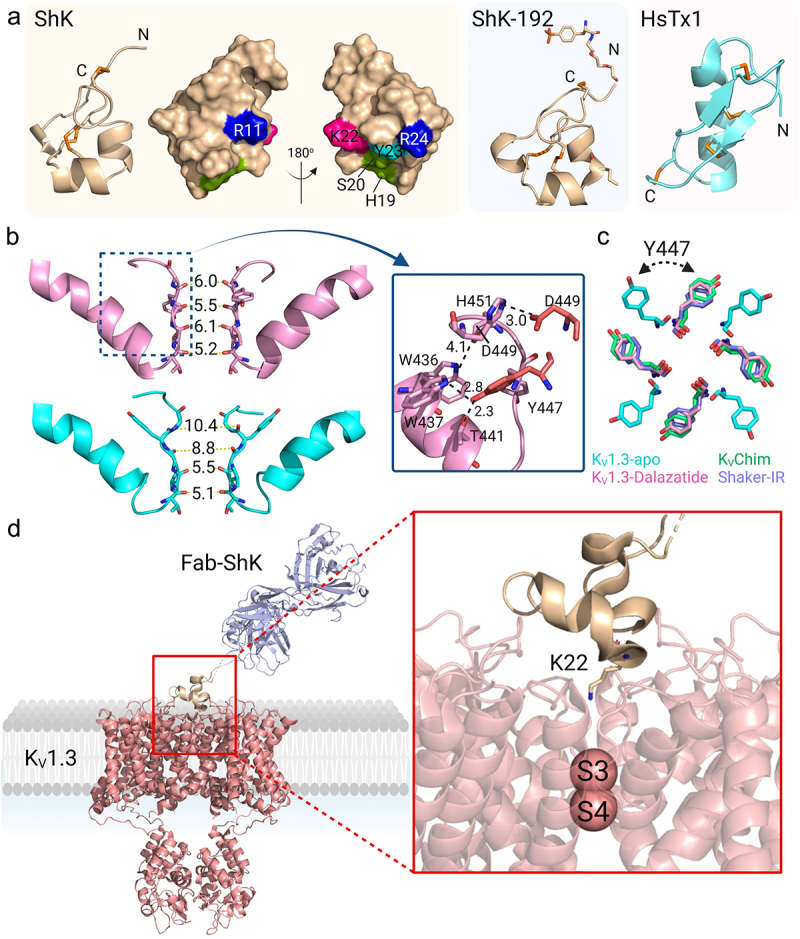
Structure of KV1.3 bound to pore-blocking peptides. a) KV1.3 inhibitory peptides. ShK represented as wheat-colored cartoon with the disulfide-bonds as orange sticks. The cluster of residues that interact with KV1.3 are represented as a surface: Arg11 and Arg24 in blue, His19 and Ser20 in green, Lys22 in pink, Tyr23 in cyan. ShK-192 (PDB 2K9E) is shown as a wheat-colored cartoon (middle) and HsTx1 (PDB 1QUZ) as a cyan-colored cartoon. b) Pore region of two subunits of the C-type inactivated apo KV1.3-KVβ2 (cyan) (PDB 7WF3) and dalazatide-KV1.3-KVβ2 (pink) (PDB 7WF4). The selectivity filter residues are shifted compared to the apo KV1.3-KVβ2, where the new position of Tyr447 formed inter-subunit hydrogen bonds Trp437-Tyr447 and Thr441-Tyr447. An inter-subunit Asp449-His451 hydrogen bond also stabilizes this conformation of the selectivity filter. c) Overlay of aromatic residues in the open-conducting conformations of KVChim (green), Shaker-IR (purple), apo KV1.3-KVβ2 (cyan) and dalazatide-KV1.3-KVβ2 (pink). d) KV1.3 bound to Fab-ShK (PDB 7SSV) with Lys22 inserted into the selectivity filter to occlude the channel pore.
