Abstract
Numerous studies have shown that hepatocyte transplantation is a promising approach for liver diseases, such as liver-based metabolic diseases and acute liver failure. However, it lacks strong evidence to support the long-term therapeutic effects of hepatocyte transplantation in clinical practice. Currently, major hurdles include availability of quality-assured hepatocytes, efficient engraftment and repopulation, and effective immunosuppressive regimens. Notably, cell sources have been advanced recently by expanding primary human hepatocytes by means of dedifferentiation in vitro. Moreover, the transplantation efficiency was remarkably improved by the established preparative hepatic irradiation in combination with hepatic mitogenic stimuli regimens. Finally, immunosuppression drugs, including glucocorticoid and inhibitors for co-stimulating signals of T cell activation, were proposed to prevent innate and adaptive immune rejection of allografted hepatocytes. Despite remarkable progress, further studies are required to improve in vitro cell expansion technology, develop clinically feasible preconditioning regimens, and further optimize immunosuppression regimens or establish ex vivo gene correction-based autologous hepatocyte transplantation.
INTRODUCTION
Liver diseases take over 2 million lives annually, which accounts for 4% of all deaths worldwide1 and ranks liver disease as the 11th leading cause of death.2 OLT has been proven life-saving and is considered gold standard for patients with liver-based metabolic disorders and end-stage liver failure.3 However, there is a huge gap between the number of patients on the waiting list and the number of donor liver organs. Alternative therapies other than liver transplantation have been vigorously explored, including hepatocyte transplantation (HTx). Compared with OLT, the main advantages of HTx are that (1) the operation of HTx is easier and less invasive; (2) cells obtained from a single donor liver can be transplanted to multiple recipients; (3) cells can be cryopreserved and genetically manipulated ex vivo.
HTx, originally initiated in 1976 as a novel therapeutic modality for Crigler-Najjar syndrome type 1 and validated using hyperbilirubinic Gunn rats, has since undergone significant advances.4 The inaugural human HTx procedure was conducted in 1992, employing autologous cells for patients with cirrhotic liver diseases.5 Subsequently, in 1994, allogenic HTx utilizing fetal liver grafts was employed to manage fulminant hepatic failure.6 Over the last 30 years, >150 clinical cases of HTx have been reported.7 Based on these studies, HTx is proposed to be applied for the management of liver-based metabolic disorders and acute liver failure.
The principle of HTx for liver-based metabolic disorders is to substitute or supplement the function of abnormal hepatocytes in patients with dysfunction of a single hepatic enzyme or secretory protein, for example, Crigler-Najjar syndrome caused by the lack of activity of the hepatic enzyme bilirubin UDP-glucuronosyltransferase, urea cycle disorders resulting from abnormalities in urea cycle enzymes, and alpha-1 antitrypsin deficiency caused by the dysfunction of A1AT protease. Since the liver has remarkable excess function, a small fraction of the liver mass is often sufficient to maintain the function. In a preclinical study of HTx for the treatment of hyperphenylalaninemia, liver repopulation over 5% resulted in a significant decrease in serum phenylalanine levels.8 In addition to liver metabolic diseases, HTx could provide hepatic function for patients with acute liver failure to support the injured liver to recover or to bridge to liver transplantation. Previous studies showed that 10%-15% of the liver mass is probably required for the treatment of acute liver failure.9
Despite its promising potential, HTx faces the following obstacles, the availability of quality-assured hepatocytes, the efficiency of engraftment and repopulation, and effective immunosuppressive regimens (Figure 1). To that end, several exciting progresses have been achieved. First, in vitro primary human hepatocyte (PHH) expansion systems have been developed, in which hepatocytes can be efficiently dedifferentiated into expandable hepatic progenitor-like cells, providing an opportunity to generate quality-assured and off-the-shelf cells for HTx.10–14 In addition, recently established regimens of preparative hepatic irradiation (HIR) in combination with mitogenic stimuli promote liver repopulation efficiently in nonhuman primates and have been introduced into clinical trials for the treatment of liver-based metabolic disorders.15 After transplantation, immune rejection would be an important issue for the graft loss.15 Remarkably, several immunosuppression drugs, such as glucocorticoids,16 have been found to significantly prevent early innate immune rejection of transplanted hepatocytes. Additionally, biomarkers, such as CD40L,15 have been identified in adaptive immune-mediated rejection after allotransplantation, making it possible to monitor the graft loss. Here, we will summarize the latest findings on orthotopic HTx, evaluate the status quo of potential techniques, and forecast the future development of HTx (Table 1).
FIGURE 1.
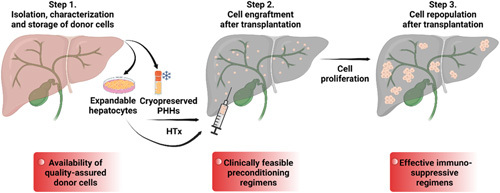
Critical procedures and barriers for HTx. Cryopreserved primary human hepatocytes (PHH) or the in vitro expanded hepatocytes are transplanted into the patient liver mainly by means of the portal vein. Due to the sinusoidal endothelium barrier, vascular injury responses and recipient immune rejection, only a small fraction of transplanted hepatocytes can successfully engraft into the host liver. Transplanted hepatocytes engraft into the host liver parenchyma, and repopulate the recipient liver in situ. To achieve HTx in clinics, 3 technical challenges should be addressed, reliable hepatocytes with quality assurance, clinically feasible preconditioning regimens to achieve cell engraftment and repopulation, and effective immunosuppressive regimens to target allograft rejection.
TABLE 1.
Hepatocyte transplantation: progresses, challenges, and outlook
| Limitations | Progress | Challenges | Future perspectives |
|---|---|---|---|
| Reliable source of transplantable cells with quality assurance | Cryopreserved PHH | The residual of DMSO Ice recrystallization Potential contamination Large-scale vitrification |
Optimization of cryoprotectant agents to replace or reduce DMSO Development of controlled rate drying warming device Optimization of the automated vitrification system |
| Pluripotent stem cells derived hepatocyte-like cells | Phenotypic immaturity Low scalability Safety issues |
Establishment of the GMP-compliant protocol for the large-scale expansion of PHH Validation of the genomic stability by whole genome sequencing Optimization the culture system to expand the adult donor hepatocytes |
|
| In vitro expansion of human hepatocytes | Safety issues GMP-compliant protocol Adult donor hepatocytes expansion |
— | |
| Clinically feasible preconditioning regimens to achieve efficient engraftment and repopulation | Drug-based and HIR-based preparative regimens to promote cell engraftment | Dose control Species sensitivity Optimal time window for transplantation |
Refinement of the engraftment process by genetic and transcriptomic tools and advanced live imaging techniques to promote engraftment |
| Preconditioning the liver to damage the host hepatocytes in combination with hepatic mitogenic stimuli regimens to promote donors’ hepatocyte repopulation | Dose of HIR Portal embolization Appropriate hepatotoxins Selection of hepatic proliferation agents |
Establishment of appropriate animal models that mimic the human liver response to irradiation Validation of clinically approved hepatotoxic drugs Validation of clinically approved drugs that stimulate hepatic proliferation, such as T3 mimetic drugs |
|
| Effective immune-suppressive regimens to allow the long-term survival of transplanted cells | Adaptive immune-mediated rejection (rejection biomarker: CD154 and CD47) | Monitoring of cellular graft function and rejection Long-term maintenance of the graft survival |
Optimization of noninvasive 3D imaging and identification of sensitive biomarkers to monitor long-term rejection Autologous HTx based on ex vivo gene correction to cure metabolic liver diseases |
| Innate immune-mediated rejection (potential treatment: A1AT and dexamethasone) | Perfusion/reperfusion injury and IBIMR during PHH transplantation Upregulation of DAIFs during long-term expandable PHH |
— |
Abbreviations: 3D, 3-dimensional; DAIFs, dedifferentiation-associated inflammatory factor; IBMR, instant blood-mediated inflammatory reaction; PHH, primary human hepatocyte; HIR, hepatic irradiation; HTx, hepatocyte transplantation
TECHNICAL CHALLENGES OF HTx
Cell source and quality
The acquisition of transplantable hepatocytes is the prerequisite for HTx and strongly associated with clinical outcomes. Due to the shortage of donor organs, PHH used in clinical HTx are primarily obtained from unused segments of donor liver tissues,17 which usually do not meet the need for OLT. On top of that, cell isolation from liver tissue includes collagenase perfusion, cell isolation and purification, and the quality of isolated hepatocytes largely depends on the quality of donor organs, making the process difficult to control and causing large variations from batch to batch.18 Moreover, cryopreserved hepatocytes do not recover well after thawing, often accompanying with low viability and impaired hepatic functions.19
Cell engraftment and repopulation
Another technical challenge of HTx is the low efficiency of cell engraftment and repopulation. Hepatocytes are mainly transplanted by means of the portal vein or spleen pulp. Transplanted cells initially move toward the branches of the portal vein and later translocated into the hepatic sinusoids, eventually integrating into the liver parenchyma. Due to the sinusoidal endothelium barrier and initial vascular responses, only a small fraction of transplanted hepatocytes can successfully engraft into the host liver.20,21 In addition, these engrafted hepatocytes encounter other difficulties in long-term retention and repopulation. For example, the host liver architecture is usually intact in patients with liver-based metabolic disorders. Also, transplanted hepatocytes would not survive for a long time in the host liver unless they possess competitive advantages of proliferation over host hepatocytes. As a result, only short-term hepatic support has been achieved after transplantation.6,22–29 It is essential to establish clinically feasible preconditioning regimens to promote cell engraftment and repopulation for successful HTx.
Immune rejection
Immune rejection is another constraint to the therapeutic application of HTx. PHH appear to be highly immunogenic after transplantation, especially in allogeneic transplantation.30 Transplanted cells are acutely rejected by adaptive (mainly mediated by T cells)31,32 and innate (eg, KC and neutrophils) immune responses.33 Transplanted cells are eliminated by adaptive immune cells, including both CD4+ and CD8+ T cells, in a short period.32 T cell–mediated rejection and more specifically CD4+ T cell–mediated rejection is well known from solid organ transplantation models.34 However, recent studies have indicated the dominant role of CD8+ T cells in hepatocyte rejection due to MHC class I–specific alloreactivity. In the clinical study of HTx in a patient with Crigler-Najjar syndrome type I, it was revealed that graft function was progressively lost due to intense CD8+ T cell alloreactivity, specifically directed toward particular HLA class I allogeneic antigens.31
In addition to adaptive immune rejection, preclinical studies have shown that the innate immune response is activated by several possible mechanisms, such as exposure of hepatocyte adhesion surface molecules that are not accessible to macrophages and neutrophils in intact tissues.33,35 Moreover, instant blood-mediated inflammatory reaction (IBMIR)36 and microcirculation disturbances caused by cell transplantation, such as transient ischemia-reperfusion injury,21 were proposed to remarkably activate KCs. These innate immune responses are often accompanied with highly increased local vasoactive molecules (eg, NO, prostacyclin, platelet-associated thrombogenic substances, endothelin, and cyclooxygenase), cytokines (eg, TNF α and IL-6), and chemokines (eg, Cxcl1, Cxcl2, Ccl3, and Ccl4).37
Currently, no consensus has been reached on the optimal immunosuppressive protocol for clinical HTx. Most centers adopt the cocktail used for solid organ transplantation to maintain immunosuppression. Steroids and calcineurin inhibitors are often applied to induce an immunosuppressive status, including methylprednisolone, tacrolimus, or cyclosporine. Other monoclonal antibodies, such as anti-interleukine-2 receptor antibodies and antilymphocyte antibodies,15 have also been included by some centers. Drugs targeted T cells were also applied, such as methylprednisolone, tacrolimus, mycophenolate mofetil, and prednisone.15 However, long-term rejection of PHH after transplantation has not yet been solved using these immunosuppressive regimens.
THE UPDATES OF TECHNOLOGY ADVANCES
Cell source for HTx
Primary human hepatocyte
Until now, PHH isolated by standardized 3-step collagenase perfusion are the major cell source used in clinical studies.38 Only quality-assured cells (sterile and viability >70%) can be applied for transplantation; however, cryopreservation of PHH negatively affects the viability and metabolic function of hepatocytes.19 PHH are typically frozen in University of Wisconsin solution with 10%–15% DMSO and 5% glucose.19 Recently, modifications have been reported to improve the quality of cryopreserved PHH, such as encapsulation of hepatocytes,39 alternative cryopreservation media to University of Wisconsin solution,40 the addition of membrane stabilizer41 and antioxidative chemicals (myricetin),42 and bulk droplet vitrification.43 Despite these improvements, the variability between different batches of PHH remains a major challenge.
Alternative cell sources
To date, many protocols have been developed to generate human hepatocytes, based on the understanding of the embryonic development of the liver and modulating the stage-specific cell signaling pathway.44 In addition, hepatocytes could be generated directly from fibroblasts by means of overexpression of liver-enriched transcription factors.45–47 Recent studies focused on 3-dimensional (3D) culture systems to induce mature hepatocytic functions and to scale up the culture system, including nonadherent plates, agarose micromold technology, and spinner flask system.48–50 Standardized GMP-compliant protocols have also been developed to generate iPSC-derived hepatocytes.51 Nevertheless, additional efforts are required to further improve mature hepatic functions and transplantation efficiency for hepatocytes derived from both strategies.
Large-scale production of human hepatocytes through in vitro expansion
Recent studies using rodent models found that mature hepatocytes could be reprogrammed into liver progenitor-like cells in vivo after chronic periportal liver injury.52–58 Inspired by these findings, several groups screened candidate growth factors and bioactive small molecules to expand primary rodent hepatocytes from the view of reprogramming.59,60 The criteria for the candidate factors were based on their ability to (1) activate specific cytokine-mediated and growth factor–mediated pathways involved in regulating liver regeneration; (2) mimic Wnt signals which is essential for liver replenishment and the activation of progenitor-like cells after liver damage; (3) inhibit TGF-β to promote proliferation and block apoptosis for the long-term cell expansion. The expansion system was successfully established to convert mature rodent hepatocytes in vitro into progenitor-like cells with a repopulation capacity using combinations of Wnt agonists, TGF-β inhibitors, and ROCK inhibitors.59,60
Importantly, several groups independently developed culture systems to dedifferentiate PHH into progenitor-like cells in vitro,10–14 and these cells could be expanded for multiple passages in large quantities. Among these studies, Zhang et al10 defined a culture medium containing Wnt3a to induce human hepatocytes to enter a bi-phenotypic state and named these cells proliferating human hepatocytes (ProliHHs). ProliHHs could be passaged for more than 1 month with a 10,000-fold increase in number. Importantly, ProliHHs at early passages showed repopulation capacity and therapeutic efficacy comparable to those of PHHs when Fah-deficient mice were used as transplantation recipients.10 Zhang and colleagues and others found that these expandable hepatocytes only maintained mature hepatic function within certain passages in vitro.10–14 To address the decreased hepatic function on passaging, Zhang and colleagues and others defined a 3D organoids culture system to facilitate the expandable hepatocytes regain mature features.10–12 In addition, Xiang et al61 reported that 2-dimensional hepatocytes maintain mature hepatic function in vitro by modulating cell signaling pathways with a combination of the adenylate cyclase activator (forskolin), TGF-β inhibitor (SB431542), Notch inhibitor (DAPT), Wnt inhibitor (IWP2), and BMP inhibitor (LDN193189). Regarding to the gradually decreased repopulation capacity of expandable hepatocytes,14,16 Wang et al16 recently found that ProliHHs cultured in 3D organoids showed increased transplantation efficiency in vivo, likely due to the reduced expression of dedifferentiation-associated inflammatory factors. The development and application of these expandable hepatocytes could partially resolve the PHH shortage. Markedly, expandable hepatocytes can be quality-assured during the expansion to meet the requirements of off-the-shelf products, and these cells provide the opportunity to develop ex vivo gene corrections for autologous HTx.
Preconditioning regimens for successful HTx
Drug- and HIR-based preparative regimens to promote cell engraftment in animals
After the transplanted hepatocytes are infused into the recipient liver, they enter the sinusoids and pass into the liver parenchyma. During these steps, 70%–80% of the initially transplanted cells are acutely cleaned due to IBMIR and inflammatory response toxicity, which results in poor engraftment efficiency.20,21,37 Pretreatment with anti-inflammatory drugs, such as thalidomide and etanercept (TNFα antagonist), 62 has been applied prior to cell transplantation (Figure 2), which greatly ameliorated inflammation and improved hepatocyte engraftment in rats.
FIGURE 2.
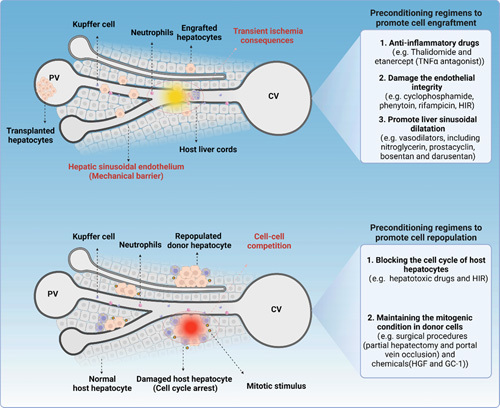
Preconditioning regimens for successful HTx. Preclinical research in preconditioning regimens for successful HTx focuses on improving the initial engraftment and long-term repopulation. In terms of enhancing engraftment, the current strategies mainly involve the use of anti-inflammatory drugs (eg, thalidomide and etanercept), to reduce ischemia-reperfusion injury-mediated inflammatory response and toxicity, as well as the utilization of drugs (eg, cyclophosphamide, phenytoin, and rifampicin) or low-dose HIR to directly disrupt the integrity of liver sinusoidal endothelium. In addition, several vasodilators (eg, nitroglycerin, prostacyclin, bosentan, and darusentan), which promote liver sinusoidal dilatation and permit transplanted cells to move through, remarkably increase the number of engrafted cells. Regarding the promotion of repopulation, the current primary concept is to block the cell cycle of host hepatocytes while maintaining a mitogenic condition in donor cells. In terms of blocking the cell cycle, it mainly involves the use of certain hepatotoxic drugs (eg, cyclophosphamide, phenytoin, and rifampicin) or high-dose HIR to induce hepatocytes senescence. To stimulate donor cell proliferation, surgical procedures (eg, partial hepatectomy and portal vein occlusion) and small molecules (eg, HGF and GC-1) can currently be used to promote hepatocytes proliferation. Under these conditions, transplanted hepatocytes gain a proliferative advantage and gradually replace the host hepatocytes.
In addition, chemical drugs have been developed to damage the endothelial integrity to improve the entrance of transplanted hepatocytes, including cyclophosphamide, phenytoin, and rifampicin63,64 (Figure 2). At the appropriate dose, these chemicals only damage liver sinusoidal endothelial cells without hepatocellular toxicity. The application of these drugs has been demonstrated to greatly improve the efficiency of hepatocyte engraftment in rodent models. In addition, several vasodilators, including nitroglycerin, prostacyclin, bosentan, and darusentan,64–66 which promote liver sinusoidal dilation and permit transplanted cells to move through, remarkably increase the number of engrafted cells (Figure 2).
Furthermore, a preparative regimen of HIR was developed to improve engraftment efficiency by destroying hepatic sinusoidal endothelium integrity (Figure 2). Notably, low-dose HIR treatment in defined liver lobes was proven safe in clinical applications,15 and the injury induced by HIR was reversible.67 The engraftment efficiency was HIR dose-dependent within a specified range. Remarkably, HIR-enhanced engraftment was validated in cynomolgus monkeys, showing that a single 10 Gy dose of HIR was sufficient to enhance engraftment of donor porcine hepatocytes.67 In summary, current drug-based or HIR-based preconditioning regimens have shown promising effects in improving cell engraftment. Importantly, preclinical animal studies have demonstrated the safety and feasibility of these regimens.
Preparative HIR in combination with hepatic mitogenic stimuli regimens to promote cell repopulation in animals
The impressive liver repopulation achieved under genetic selection conditions, such as the urokinase plasminogen activator transgenic mice or Fah-deficient mice, provides clues for the optimization of the preconditioning regimen to improve donor cell repopulation, that is, blocking the cell cycle of host hepatocytes while maintaining the mitogenic condition in donor cells (Figure 2). Previously, retrosine, a pyrrolizidine alkaloid, was administered to inhibit the regeneration of host hepatocytes in rats. Thus, only the donor cells gained the advantage of proliferation in response to a mitotic stimulus induced by partial hepatectomy.68 However, retrosine is not suitable for clinical use because of the genotoxicity and tumorigenic risk. Later, Guha et al69–71 found that the combination of HIR and hepatotropic growth stimuli successfully enabled high repopulation efficiency in animals with inherited metabolic liver diseases. Notably, HIR can be accurately delivered into the specific lobe of the host liver in a spatially confined manner, leaving the remaining liver unperturbed.72
In addition to HIR, other preconditioning protocols have been developed to identify regimens that induce controllable damage to hepatocytes. Loss of Cypor would abrogate Cyp-mediated metabolism, which prevents the conversion of acetaminophen to hepatotoxic NAPQI. Vonada et al73 reported that hepatocytes lacking Cypor would be protected from acetaminophen-induced toxicity. They also showed that with the transient treatment of moderately hepatotoxic acetaminophen, hepatocytes lacking Cypor were selectively enriched in vivo. By linking therapeutic transgenes in cis with a CRISPR-Cas9 guide RNA or a short hairpin RNA targeting Cypor, hepatocytes with reduced Cypor expression could be efficiently repopulated with APAP treatment, replacing up to 50% of the liver mass in the preclinical animal studies. It is thus possible to expand hepatocytes harboring a disease-curing transgene to therapeutic levels after transplantation.73
Another challenge for preconditioning regimens is to promote the proliferation of transplanted cells. To this end, surgical procedures and chemicals have been investigated as potential mitotic stimuli (Figure 2). Although partial hepatectomy and portal vein occlusion are routine clinical operations, the regenerative stimuli induced by these manipulations are short-acting. Several chemicals have been validated as hepatic mitogens that promote hepatocyte regeneration, including HGF,67,72,74,75 the thyroid hormone, triiodothyronine (T3),68,76 and GC-1 (a T3 mimetic drug)72 (Figure 2). However, recombinant HGF is unstable in blood with a half-life of 3–5 minutes,77–80 while virus-mediated expression of HGF raises safety issue.81,82 Additional safety concerns for HGF include negative effects on cardiac function and off-target effect as multiple tissues express HGF receptor.83,84 Regarding T3, the effective dose of T3 induces severe cardiotoxicity because T3 can directly bind to the receptor TRα in cardiac tissues. Although the T3 mimetic GC-1 is nontoxic to cardiomyocytes and efficient in promoting hepatocyte regeneration, it has not yet been approved by the Food and Drug Administration (FDA). Another T3 mimetic, resmetirom (MGL-3196), which received breakthrough therapy designation from FDA,85 could be additionally evaluated to promote hepatocyte proliferation at a safe dosage.
In general, preparative protocols based on HIR in combination with hepatic mitogenic agents may be applied in clinical trials in the future. Nevertheless, additional efforts are expected to improve clinically compatible preconditioning regimens, especially the optimization of HIR and selection of hepatotoxins and hepatic proliferation agents.
Clinical HTx studies under HIR-based preparative regimens
Recently, Soltys et al15 performed the clinical trial to implement preparative HIR protocols in 3 patients with inherited metabolic liver diseases. Two young infants with urea cycle defects received preparative HIR with a single dose of 5 and 7.5 Gy, respectively, targeting 30%–37% of the total liver volume prior to HTx. Unfortunately, they experienced early graft loss after HTx, and a retrospective analysis attributed this to inadequate immunosuppression. In another case, a female adult with phenylketonuria received a single fraction of preparative HIR at 10 Gy. Approximately 3% donor cells in the recipient liver biopsies were detected 3–6 months after HTx. Furthermore, the serum phenylalanine level was ~36% lower than before HTx.15 The initial results from these 3 patients demonstrated the safety of low-dose preparative HIR preconditioning of the liver. However, the efficacy of HTx in patients differs significantly from preclinical data.15 It is essential to further investigate various aspects, such as irradiation dose and volume, the quality and dose of transplanted cells, and immunosuppression protocol.
Progress in preventing immune rejection of HTx
Recent studies have shown that the immune response to HTx is different from that to OLT.86 Allograft complications are regularly monitored during organ transplantation, and standard clinical and laboratory measures have been developed to identify organ rejection, including cell-mediated and antibody-mediated rejection and manipulation tolerance, all of which contribute to the management of immunosuppression in transplant recipients.87 At present, the clinical immunosuppressive regimens for HTx are derived from protocols applied in OLT, even though hepatocytes are highly immunogenic compared with liver transplants. These immunosuppressive protocols mainly suppress the adaptive immune response by inhibiting the activity of T cells.15 In contrast to OLT, it is currently difficult to monitor the fate, location, and number of transplanted cells in the recipient liver, necessitating the evaluation of a potential rejection marker during HTx.
Previously, mice were treated with either anti-CD40L (also known as CD154) antibody or anti-CTLA4 antibody to block CD40L/CD40 or CD28/B7 signaling, respectively. Anti-CD40L antibody caused significant enhanced survival of allografted hepatocytes, whereas the application of CTLA4 antibody showed no obvious effects.88 Further, it was identified that treatment of CD8-knockout or CD4-knockout mice with anti-CD40L antibody led to significantly prolonged survival of transplanted hepatocytes, indicating that CD40L/CD40 interaction was also critical in both CD4+ and CD8+ T cell–mediated rejection. Importantly, a recent clinical HTx study found that donor-specific alloreactivity could be monitored by detecting allograft-specific CD40L+ cytotoxic memory T cells, which may be a potential biomarker for monitoring early rejection by adaptive immune cells after HTx.15
In addition, previous studies have identified the role of neutrophils and macrophages in the early elimination of transplanted cells.33 Moreover, depletion of neutrophils and KCs significantly improved cell engraftment in rats.33 At present, perfusion/reperfusion injury induced by embolus of transplanted hepatocytes and IBMIR has been proposed responsible for the activation of the innate immune response after HTx, mainly KC activation.86 These processes are accompanied by the release of numerous deleterious proinflammatory cytokines, leading to cellular rejection. Lee and colleagues recently reported that administration of A1AT, which is a natural immunomodulator secreted by hepatocytes that inhibits caspase-related inflammation,89,90 improved engraftment of transplanted hepatocytes by inhibiting IBMIR.91,92 In addition, Yang and colleagues found that CD47, a member of the immunoglobulin superfamily that provides a protective signal against the phagocytic activity of macrophages, regulated both innate and adaptive immune-mediated rejection of transplanted hepatocytes.93
These studies mainly focused on immune rejection after primary hepatocytes, which receive minimal manipulation after isolation. However, cells produced by means of stem cell technology usually require considerable long-term culture in vitro and the molecular expression pattern might be altered after passaging.94,95 Wang et al16 recently reported immune rejection of expandable human hepatocytes, ProliHH. In correlation with the reduced transplantation efficiency of long-term cultured ProliHH (lc-ProliHH), it was found that dedifferentiation-associated inflammatory factors were upregulated in lc-ProliHH. Further analysis showed that innate immune cells, specifically Kupffer cells and neutrophils, were rapidly activated by lc-ProliHH after transplantation, which resulted in their clearance.
FUTURE PERSPECTIVES
Cell source for HTx
While 10% DMSO has been widely used for the PHH cryopreservation, alternative cryoprotectant agents should be studied to replace or reduce DMSO. Moreover, issues regarding vitrification requires further analyses, including large-scale vitrification, potential cell loss caused by the recollection of frozen cells, and cell functionality in vitrificated PHH. Due to the challenges in maturation and scale up, the application of iPSC-derived hepatocytes is mainly reported in animal models. With new progress in these directions, the clinical application of iPSC-derived hepatocytes would be foreseeable. Currently, the quantity of PHH using different expansion protocols can theoretically meet clinical needs. It is necessary to ensure genetic stability and in vivo safety of these hepatocytes before they can be used in clinical applications. Nevertheless, advancements in terms of maturity, functional stability, and GMP production are required for either iPSC-derived hepatocytes and expanded PHH. For clinical research, a GMP-compliant protocol should be established for the expansion of these cells in large quantities. Reagents used during the culture, including the basic culture medium and other additional supplements, need to be optimized to be compliant with GMP and reduce unknown effects on humans.
Currently, available PHH expansion systems perform best with hepatocytes obtained from pediatric donors. Although some adult hepatocytes are expandable for a few passages, it remains challenging to expand hepatocytes from all adult donors in large scale.10–14 The limited proliferation of adult hepatocytes should be investigated by identifying the differentially expressed genes and pathways between pediatric and adult donor cells. Importantly, small molecules targeting these genes and pathways can be screened to promote the proliferation of adult hepatocytes, especially chemicals targeting epigenetic modifications and cellular senescence. In addition, strategies may be developed by analyzing pathways controlling liver regeneration. It is noteworthy that hepatocyte expansion not only refers to hepatocyte proliferation in vitro, but also requires these expandable hepatocytes maintaining mature hepatic function. To that end, potential chemical combinations and 3D organoid culture that maintain the function of PHH could be applied to help expandable hepatocytes regain mature features.
HTx technology
Cell engraftment and subsequent repopulation is critical for successful HTx. Current studies focus on changes in the host liver microenvironment during transplantation, and existing preconditioning regimens were designed based on this direct. However, the underlying mechanisms of engraftment and repopulation remain largely elusive, including how transplanted cells survive, migrate, integrate, and repopulate in the host liver. The development of multiple genetic tools and advanced live imaging techniques has enabled researchers to trace the fate of transplanted cells in host livers. By combining cell sorting with transcriptomic analysis, transplanted cells can be identified and captured to study cell behavior at different stages in the recipient liver. New knowledge will promote engraftment and proliferation of transplanted hepatocytes.
Current preconditioning regimens, especially preparative HIR, have demonstrated long-term therapeutic potential in preclinical animal models of liver-based metabolic diseases. Moreover, they have been deemed safe and supportive of engraftment in clinical settings. However, the observed level of replacement of donor hepatocytes has fallen short of expectations. This disparity in clinical efficacy is mainly attributed to radiation conditions (dose and volume), variations in hepatic radiosensitivity across species, donor cell quality, timing of transplantation, and immunosuppressive regimens. The eventual clinical application of these methods hinges on meticulous preclinical investigations conducted in animal models and a cautious, incremental development of protocols for human subjects.
Notably, hepatic conditioning regimens inherently entail hepatotoxicity and may entail substantial side effects, similar as the myeloablation associated with bone marrow transplantation, which is not completely innocuous. Therefore, it is critical to evaluate the potential liver damage of preparative HIR preconditioning regimens to enable the appropriate target patients to benefit from this therapy. Given that HIR activates stellate cells, careful consideration should be given to avoid the inclusion of patients with cirrhosis and for patients with severe liver damage. Besides, safety risks associated with liver irradiation in infant patients need to be meticulously evaluated. The preferred candidates for this procedure would be those with diseases that allow for easy measurement of the therapeutic effects of transplanted cells and individuals who would require an organ transplant if the surgery is unsuccessful.
Given that a single dose of HIR injury is unlikely to cause immediate cell death, alternative irradiation techniques, such as the implantation of radioactive particles, can be explored. These methods would enable the administration of repeated low-dose HIR, resulting in the expected damage. In addition, some clinically approved chemotherapeutics (eg, PARP inhibitors) that inhibit DNA damage repair, could be combined with HIR to efficiently induce the cell death of host hepatocytes, thereby permitting selective growth of the undisturbed donor cells. Finally, it would be interesting to develop personalized preconditioning regimens, for example, host hepatocytes may be sensitive to a specific drug or HIR in certain liver diseases.
Strategies to improve immune rejection in HTx
It is important to comprehensively understand the kinetics of immune rejection during the whole process of HTx. Hepatocytes are known to express major histocompatibility complex class I, adhesion molecules, and co-stimulation molecules, such as CD40, which is a marker of T cell activation associated with graft rejection. Gao et al96 reported that human hepatocytes induced T cell activation by means of CD40/CD40L in vitro, suggesting that the surface markers of hepatocytes may be changed during the isolation or culture. The protection of isolated cells from T cells may mitigate immune rejection. Hepatocytes can be incubated with anti-CD40 antibodies or pretreated with protective matrices like sodium hyaluronate. After delivering into the parenchyma, it is crucial to optimize anti-inflammatory drugs to prevent the occurrence of IBMIR and innate immune rejection triggered by ischemic injury.
Long-term immunosuppressive intervention is employed to ensure the prolonged survival of transplanted hepatocytes. For this purpose, an effective immune rejection monitoring system needs to be established, such as noninvasive 3D imaging. Currently, the rejection risk after allogeneic HTx can be evaluated by monitoring CD40L+ T cells targeting donor cells.15 Based on the monitored immune reactivity, combinatorial protocols to induce and maintain immunosuppression would be thus designed to control cell-mediated allograft rejection. Further studies should be performed to identify more efficient immunosuppressant regimes to target T cells. For example, drugs that block CD40L/CD40 co-stimulatory signaling need to be developed. It is noteworthy that several clinical trials of CD40/CD40L monoclonal antibodies are currently under investigation in renal transplantation and Sjogren syndrome.97 Moreover, given the reported role of CD8+ T cell–-mediated rejection in HLA class I–specific allograft rejection,31 donor-specific antibody-driven rejection should be considered.
In terms of expanded PHH transplantation, Wang et al16 demonstrated an unexpected role of macrophages in preventing the engraftment of long-term cultured ProliHH. Interestingly, when remature in organoid cultures, lc-ProliHH successfully reduced their ability to recruit and activate macrophages and greatly improved the transplantation efficiency. Alternatively, the transplantation efficiency of lc-ProliHH was significantly improved by macrophage depletion in the recipients. Moreover, when recipients were treated with glucocorticoids to suppress innate immune responses, engraftment efficiency was significantly enhanced.16 It appears to be important to target macrophages and to identify the most essential cytokines for the development of immunosuppressants for clinical transplantation of expanded PHH.
Autologous cell transplantation therapy with ex vivo gene correction may be another potential and efficient therapy for liver-based metabolic disorders. Recently, Zhang et al98 found ProliHHs were more susceptible to gene manipulation compared with PHH and demonstrated the efficacy of factor VIII-modified ProliHHs in hemophilia A mouse model. With further studies on promoting engraftment and repopulation efficiency, the combination of ex vivo gene editing with in vitro expansion of autologous hepatocytes could eventually replace mutated hepatocytes to cure metabolic liver diseases.
Footnotes
Abbreviations: 3D, 3-dimensional; DAIFs, dedifferentiation-associated inflammatory factor; FDA, Food and Drug Administration; GMP, good manufacturing practice; HIR, hepatic irradiation; HTx, hepatocyte transplantation; IBMIR, instant blood-mediated inflammatory reaction; lc-ProliHH, long-term cultured; NO, nitric oxide; ProliHH; PHH, primary human hepatocyte; ProliHHs, proliferating human hepatocytes; T3, triiodothyronine.
Zhen Sun, Xiang Yuan, and Jingqi Wu contributed equally.
Contributor Information
Zhen Sun, Email: sunzh@shanghaitech.edu.cn.
Xiang Yuan, Email: yuanxiang2013@sibcb.ac.cn.
Jingqi Wu, Email: wujingqi2019@sibcb.ac.cn.
Chenhua Wang, Email: wangchenhua2017@sibcb.ac.cn.
Kun Zhang, Email: kun.zhang@sibcb.ac.cn.
Ludi Zhang, Email: zhangludi@sibcb.ac.cn.
Lijian Hui, Email: ljhui@sibcb.ac.cn.
ACKNOWLEDGMENTS
The authors thank Prof. Yun-Wen Zheng (University of Tsukuba, Japan), the laboratory members and anonymous reviewers for critical comments and suggestions, which improved this manuscript significantly.
FUNDING INFORMATION
This project was supported by the “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA16020201), the National Key Research and Development Project (2020YFA0112503), and the National Natural Science Foundation of China (NSFC) (32070797).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
REFERENCES
- 1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–71. [DOI] [PubMed] [Google Scholar]
- 2. Griffin C, Agbim U, Ramani A, Shankar N, Kanwal F, Asrani SK. Underestimation of cirrhosis-related mortality in the Medicare eligible population, 1999-2018. Clin Gastroenterol Hepatol. 2023;21:223–5 e3. [DOI] [PubMed] [Google Scholar]
- 3. Jadlowiec CC. Liver transplantation: current status and challenges. World J Gastroenterol. 2016;22:4438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Lowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976;192:892–4. [DOI] [PubMed] [Google Scholar]
- 5. Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992;24:3052–3053. [PubMed] [Google Scholar]
- 6. Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951–2. [DOI] [PubMed] [Google Scholar]
- 7. Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, Strom SC. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res. 2015;54:162–77. [DOI] [PubMed] [Google Scholar]
- 8. Hamman K, Clark H, Montini E, Al-Dhalimy M, Grompe M, Finegold M, et al. Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria. Mol Ther. 2005;12:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asonuma K, Gilbert JC, Stein JE, Takeda T, Vacanti JP. Quantitation of transplanted hepatic mass necessary to cure the Gunn rat model of hyperbilirubinemia. J Pediatr Surg. 1992;27:298–301. [DOI] [PubMed] [Google Scholar]
- 10. Zhang K, Zhang L, Liu W, Ma X, Cen J, Sun Z, et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell. 2018;23:806–19 e4. [DOI] [PubMed] [Google Scholar]
- 11. Hu H, Gehart H, Artegiani B, LO-I C, Dekkers F, Basak O, et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175:1591–606 e19. [DOI] [PubMed] [Google Scholar]
- 12. Fu GB, Huang WJ, Zeng M, Zhou X, Wu HP, Liu CC, et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019;29:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim Y, Kang K, Lee SB, Seo D, Yoon S, Kim SJ, et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol. 2019;70:97–107. [DOI] [PubMed] [Google Scholar]
- 14. Katsuda T, Matsuzaki J, Yamaguchi T, Yamada Y, Prieto-Vila M, Hosaka K, et al. Generation of human hepatic progenitor cells with regenerative and metabolic capacities from primary hepatocytes. Elife. 2019;8:e47313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soltys KA, Setoyama K, Tafaleng EN, Soto Gutierrez A, Fong J, Fukumitsu K, et al. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol. 2017;66:987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang C, Zhang L, Sun Z, Yuan X, Wu B, Cen J, et al. Dedifferentiation-associated inflammatory factors of long-term expanded human hepatocytes exacerbate their elimination by macrophages during liver engraftment. Hepatology. 2022;76:1690–705. [DOI] [PubMed] [Google Scholar]
- 17. Hughes RD, Mitry RR, Dhawan A, Lehec SC, Girlanda R, Rela M, et al. Isolation of hepatocytes from livers from non-heart-beating donors for cell transplantation. Liver Transpl. 2006;12:713–7. [DOI] [PubMed] [Google Scholar]
- 18. Li AP. Human hepatocytes: isolation, cryopreservation and applications in drug development. Chem Biol Interact. 2007;168:16–29. [DOI] [PubMed] [Google Scholar]
- 19. Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transpl. 2010;16:229–37. [DOI] [PubMed] [Google Scholar]
- 20. Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. Studies of liver repopulation using the dipeptidyl peptidase IV-deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology. 1996;23:482–96. [DOI] [PubMed] [Google Scholar]
- 21. Gupta S, Rajvanshi P, Sokhi R, Slehria S, Yam A, Kerr A, et al. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999;29:509–19. [DOI] [PubMed] [Google Scholar]
- 22. Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–6. [DOI] [PubMed] [Google Scholar]
- 23. Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111(6 Pt 1):1262–7. [DOI] [PubMed] [Google Scholar]
- 24. Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–8. [DOI] [PubMed] [Google Scholar]
- 25. Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812–4. [DOI] [PubMed] [Google Scholar]
- 26. Mas VR, Maluf DG, Thompson M, Ferreira-Gonzalez A, Fisher RA. Engraftment measurement in human liver tissue after liver cell transplantation by short tandem repeats analysis. Cell Transplant. 2004;13:231–36. [DOI] [PubMed] [Google Scholar]
- 27. Stephenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058–61. [DOI] [PubMed] [Google Scholar]
- 28. Puppi J, Tan N, Mitry RR, Hughes RD, Lehec S, Mieli-Vergani G, et al. Hepatocyte transplantation followed by auxiliary liver transplantation--a novel treatment for ornithine transcarbamylase deficiency. Am J Transplant. 2008;8:452–7. [DOI] [PubMed] [Google Scholar]
- 29. Meyburg J, Das AM, Hoerster F, Lindner M, Kriegbaum H, Engelmann G, et al. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation. 2009;87:636–41. [DOI] [PubMed] [Google Scholar]
- 30. Bumgardner GL, Orosz CG. Unusual patterns of alloimmunity evoked by allogeneic liver parenchymal cells. Immunol Rev. 2000;174:260–79. [DOI] [PubMed] [Google Scholar]
- 31. Allen KJ, Mifsud NA, Williamson R, Bertolino P, Hardikar W. Cell-mediated rejection results in allograft loss after liver cell transplantation. Liver Transpl. 2008;14:688–94. [DOI] [PubMed] [Google Scholar]
- 32. Bumgardner GL, Heininger M, Li J, Xia D, Parker-Thornburg J, Ferguson RM, et al. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation. 1998;65:53–61. [DOI] [PubMed] [Google Scholar]
- 33. Joseph B, Malhi H, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver. Gastroenterology. 2002;123:1677–85. [DOI] [PubMed] [Google Scholar]
- 34. Saitovitch D, Bushell A, Mabbs DW, Morris PJ, Wood KJ. Kinetics of induction of transplantation tolerance with a nondepleting anti-Cd4 monoclonal antibody and donor-specific transfusion before transplantation. A critical period of time is required for development of immunological unresponsiveness. Transplantation. 1996;61:1642–7. [DOI] [PubMed] [Google Scholar]
- 35. Olszewski WL, Interewicz B, Durlik M, Rudowska A, Mecner B. Early loss of transplanted autologous hepatocytes-lysis by leukocytes in vivo and in vitro. Transplant Proc. 2001;33:651–3. [DOI] [PubMed] [Google Scholar]
- 36. Gustafson EK, Elgue G, Hughes RD, Mitry RR, Sanchez J, Haglund U, et al. The instant blood-mediated inflammatory reaction characterized in hepatocyte transplantation. Transplantation. 2011;91:632–8. [DOI] [PubMed] [Google Scholar]
- 37. Krohn N, Kapoor S, Enami Y, Follenzi A, Bandi S, Joseph B, et al. Hepatocyte transplantation-induced liver inflammation is driven by cytokines-chemokines associated with neutrophils and Kupffer cells. Gastroenterology. 2009;136:1806–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitry RR. Isolation of human hepatocytes. Methods Mol Biol. 2009;481:17–23. [DOI] [PubMed] [Google Scholar]
- 39. Jitraruch S, Dhawan A, Hughes RD, Filippi C, Lehec SC, Glover L, et al. Cryopreservation of hepatocyte microbeads for clinical transplantation. Cell Transplant. 2017;26:1341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee JH, Park HJ, Kim YA, Lee DH, Noh JK, Jung JG, et al. Establishment of a serum-free hepatocyte cryopreservation process for the development of an “off-the-shelf” bioartificial liver system. Bioengineering (Basel). 2022;9:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyamoto Y, Suzuki S, Nomura K, Enosawa S. Improvement of hepatocyte viability after cryopreservation by supplementation of long-chain oligosaccharide in the freezing medium in rats and humans. Cell Transplant. 2006;15:911–9. [DOI] [PubMed] [Google Scholar]
- 42. Cui C, Enosawa S, Matsunari H, Nagashima H, Umezawa A. Natural flavonol, myricetin, enhances the function and survival of cryopreserved hepatocytes in vitro and in vivo. Int J Mol Sci. 2019;20:6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Vries RJ, Banik PD, Nagpal S, Weng L, Ozer S, van Gulik TM, et al. Bulk droplet vitrification: an approach to improve large-scale hepatocyte cryopreservation outcome. Langmuir. 2019;35:7354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gerbal-Chaloin S, Funakoshi N, Caillaud A, Gondeau C, Champon B, Si-Tayeb K. Human induced pluripotent stem cells in hepatology: beyond the proof of concept. Am J Pathol. 2014;184:332–47. [DOI] [PubMed] [Google Scholar]
- 45. Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–9. [DOI] [PubMed] [Google Scholar]
- 46. Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–3. [DOI] [PubMed] [Google Scholar]
- 47. Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–84. [DOI] [PubMed] [Google Scholar]
- 48. Yamamoto J, Udono M, Miura S, Sekiya S, Suzuki A. Cell aggregation culture induces functional differentiation of induced hepatocyte-like cells through activation of Hippo signaling. Cell Rep. 2018;25:183–98. [DOI] [PubMed] [Google Scholar]
- 49. Pettinato G, Lehoux S, Ramanathan R, Salem MM, He LX, Muse O, et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with endothelial cells. Sci Rep. 2019;9:8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feng S, Wu J, Qiu WL, Yang L, Deng X, Zhou Y, et al. Large-scale generation of functional and transplantable hepatocytes and cholangiocytes from human endoderm stem cells. Cell Rep. 2020;33:108455. [DOI] [PubMed] [Google Scholar]
- 51. Blackford SJI, Ng SS, Segal JM, King AJF, Austin AL, Kent D, et al. Validation of current good manufacturing practice compliant human pluripotent stem cell-derived hepatocytes for cell-based therapy. Stem Cells Transl Med. 2019;8:124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–74. [DOI] [PubMed] [Google Scholar]
- 54. Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okabe H, Yang J, Sylakowski K, Yovchev M, Miyagawa Y, Nagarajan S, et al. Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice. Hepatology. 2016;64:1652–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li W, Yang L, He Q, Hu C, Zhu L, Ma X, et al. A homeostatic Arid1a-dependent permissive chromatin state licenses hepatocyte responsiveness to liver-injury-associated YAP signaling. Cell Stem Cell. 2019;25:54–68 e5. [DOI] [PubMed] [Google Scholar]
- 58. Li W, Li L, Hui L. Cell plasticity in liver regeneration. Trends Cell Biol. 2020;30:329–38. [DOI] [PubMed] [Google Scholar]
- 59. Katsuda T, Kawamata M, Hagiwara K, Takahashi RU, Yamamoto Y, Camargo FD, et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 2017;20:41–55. [DOI] [PubMed] [Google Scholar]
- 60. Wu H, Zhou X, Fu GB, He ZY, Wu HP, You P, et al. Reversible transition between hepatocytes and liver progenitors for in vitro hepatocyte expansion. Cell Res. 2017;27:709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiang C, Du Y, Meng G, Yi L, Sun S, Song N, et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science. 2019;364:399–402. [DOI] [PubMed] [Google Scholar]
- 62. Viswanathan P, Kapoor S, Kumaran V, Joseph B, Gupta S. Etanercept blocks inflammatory responses orchestrated by TNF-alpha to promote transplanted cell engraftment and proliferation in rat liver. Hepatology. 2014;60:1378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Slehria S, Rajvanshi P, Ito Y, Sokhi RP, Bhargava KK, Palestro CJ, et al. Hepatic sinusoidal vasodilators improve transplanted cell engraftment and ameliorate microcirculatory perturbations in the liver. Hepatology. 2002;35:1320–8. [DOI] [PubMed] [Google Scholar]
- 64. Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: from liver transplantation to cell factory. J Hepatol. 2015;62(suppl 1):S157–69. [DOI] [PubMed] [Google Scholar]
- 65. Bahde R, Kapoor S, Bandi S, Bhargava KK, Palestro CJ, Gupta S. Directly acting drugs prostacyclin or nitroglycerine and endothelin receptor blocker bosentan improve cell engraftment in rodent liver. Hepatology. 2013;57:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bahde R, Kapoor S, Viswanathan P, Spiegel HU, Gupta S. Endothelin-1 receptor A blocker darusentan decreases hepatic changes and improves liver repopulation after cell transplantation in rats. Hepatology. 2014;59:1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oren R, Dabeva MD, Karnezis AN, Petkov PM, Rosencrantz R, Sandhu JP. Role of thyroid hormone in stimulating liver repopulation in the rat by transplanted hepatocytes. Hepatology. 1999;30:903–13. [DOI] [PubMed] [Google Scholar]
- 69. Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59:5871–4. [PubMed] [Google Scholar]
- 70. Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, et al. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002;36:354–62. [DOI] [PubMed] [Google Scholar]
- 71. Guha C, Yamanouchi K, Jiang J, Wang X, Roy Chowdhury N, Santana A, et al. Feasibility of hepatocyte transplantation-based therapies for primary hyperoxalurias. Am J Nephrol. 2005;25:161–70. [DOI] [PubMed] [Google Scholar]
- 72. Barahman M, Zhang W, Harris HY, Aiyer A, Kabarriti R, Kinkhabwala M, et al. Radiation-primed hepatocyte transplantation in murine monogeneic dyslipidemia normalizes cholesterol and prevents atherosclerosis. J Hepatol. 2019;70:1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vonada A, Tiyaboonchai A, Nygaard S, Posey J, Peters AM, Winn SR, et al. Therapeutic liver repopulation by transient acetaminophen selection of gene-modified hepatocytes. Sci Transl Med. 2021;13:eabg3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou H, Dong X, Kabarriti R, Chen Y, Avsar Y, Wang X, et al. Single liver lobe repopulation with wildtype hepatocytes using regional hepatic irradiation cures jaundice in Gunn rats. PLoS One. 2012;7:e46775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen Y, Li Y, Wang X, Zhang W, Sauer V, Chang CJ, et al. Amelioration of hyperbilirubinemia in Gunn rats after transplantation of human induced pluripotent stem cell-derived hepatocytes. Stem Cell Reports. 2015;5:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Francavilla A, Carr BI, Azzarone A, Polimeno L, Wang Z, VanThiel DH. Hepatocyte proliferation and gene expression induced by triiodothyronine in vivo and in vitro. Hepatology. 1999;20:1237–41. [PubMed] [Google Scholar]
- 77. Liu KX, Kato Y, Narukawa M, Kim DC, Hanano M, Higuchi O, et al. Importance of the liver in plasma clearance of hepatocyte growth factor in rats. Am J Physiol. 1992;263:G642–9. [DOI] [PubMed] [Google Scholar]
- 78. Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–3. [DOI] [PubMed] [Google Scholar]
- 80. Liu CJ, Jones DS, II, Tsai PC, Venkataramana A, Cochran JR. An engineered dimeric fragment of hepatocyte growth factor is a potent c-MET agonist. FEBS Lett. 2014;588:4831–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT, et al. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther. 2008;16:931–41. [DOI] [PubMed] [Google Scholar]
- 82. Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473:101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ido A, Moriuchi A, Numata M, Murayama T, Teramukai S, Marusawa H, et al. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: a phase I/II clinical trial, following preclinical studies to ensure safety. J Transl Med. 2011;9:1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–24. [DOI] [PubMed] [Google Scholar]
- 86. Oldhafer F, Bock M, Falk CS, Vondran FW. Immunological aspects of liver cell transplantation. World J Transplant. 2016;6:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Castellaneta A, Thomson AW, Nayyar N, de Vera M, Mazariegos GV. Monitoring the operationally tolerant liver allograft recipient. Curr Opin Organ Transplant. 2010;15:28–34. [DOI] [PubMed] [Google Scholar]
- 88. Bumgardner GL, Li J, Heininger M, CGO. Costimulation pathways in host immune responses to allogeneic hepatocytes. Transplantation. 1998;66:1841–1845. [DOI] [PubMed] [Google Scholar]
- 89. Ikari Y, Mulvihill E, Schwartz SM. alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, and alpha 2-macroglobulin are the antiapoptotic factors of vascular smooth muscle cells. J Biol Chem. 2001;276:11798–803. [DOI] [PubMed] [Google Scholar]
- 90. Lewis EC. Expanding the clinical indications for alpha(1)-antitrypsin therapy. Mol Med. 2012;18:957–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee C, Dhawan A, Iansante V, Filippi C, Mitry R, Tang J, et al. Improving engraftment of hepatocyte transplantation using alpha-1 antitrypsin as an immune modulator. J Mol Med (Berl). 2019;97:563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee CA, Dhawan A, Smith RA, Mitry RR, Fitzpatrick E. Instant blood-mediated inflammatory reaction in hepatocyte transplantation: current status and future perspectives. Cell Transplant. 2016;25:1227–36. [DOI] [PubMed] [Google Scholar]
- 93. Zhang M, Wang H, Tan S, Navarro-Alvarez N, Zheng Y, Yang YG. Donor CD47 controls T cell alloresponses and is required for tolerance induction following hepatocyte allotransplantation. Sci Rep. 2016;6:26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Csaszar E, Kirouac DC, Yu M, Wang W, Qiao W, Cooke MP, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10:218–29. [DOI] [PubMed] [Google Scholar]
- 95. Wilkinson AC, Ishida R, Kikuchi M, Sudo K, Morita M, Crisostomo RV, et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019;571:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gao D, Li J, Orosz CG, Bumgardner GL. Different costimulation signals used by CD4(+) and CD8(+) cells that independently initiate rejection of allogenic hepatocytes in mice. Hepatology. 2000;32:1018–28. [DOI] [PubMed] [Google Scholar]
- 97. Karnell JL, Albulescu M, Drabic S, Wang L, Moate R, Baca M, et al. A CD40L-targeting protein reduces autoantibodies and improves disease activity in patients with autoimmunity. Sci Transl Med. 2019;11:eaar6584. [DOI] [PubMed] [Google Scholar]
- 98. Zhang K, Wu N, Cen J, Li J, Wang Z, Xia Q, et al. Ex vivo factor VIII-modified proliferating human hepatocytes therapy for haemophilia A. Cell Prolif. 2023;56:e13467. [DOI] [PMC free article] [PubMed] [Google Scholar]


