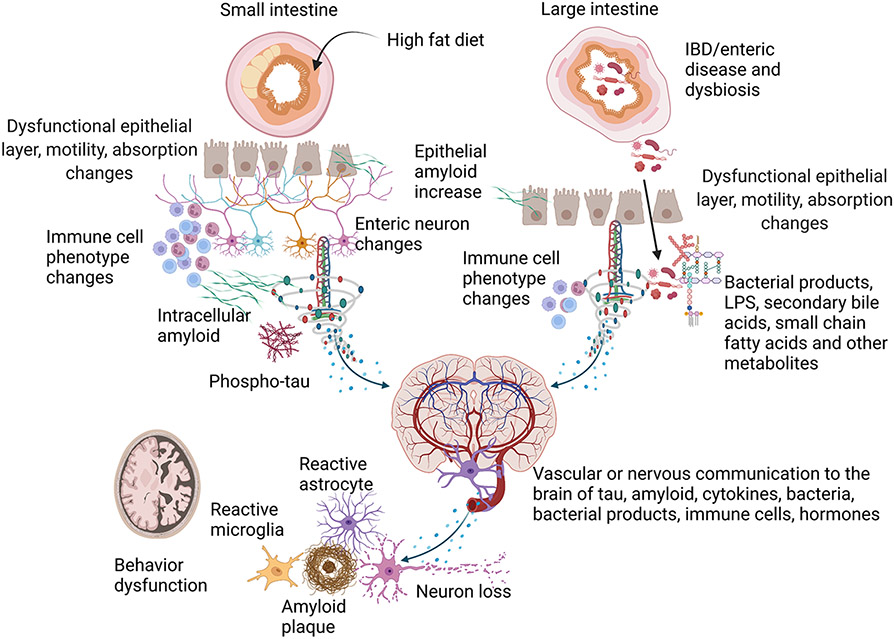
Fig. 3. Potential contributions of the small and large intestine to AD pathophysiology.
Several studies validate disease-related changes in the small intestines of AD patients and rodent models characterized by Aβ and tau pathology and inflammatory changes. For example, small intestine epithelial cells express APP in response to dietary changes such as a high-fat diet and can secrete Aβ peptide possibly into the intestinal connective tissue and the circulation. In agreement with this, enteric neurons in rodent models and AD patient small intestines have demonstrated intracellular Aβ, immune cell changes, neuron expression differences, and phospho-tau increases. Different rodent models of AD have also identified intestinal motility and permeability changes compared to controls. Although the intestine-related dysfunction represents a disease phenotype, it is possible that enteric-derived Aβ, phospho-tau, activated immune cells, enteric hormones, or cytokines may communicate to the brain to influence disease via the circulation or direct neuronal innervation. Similar contributions to disease may exist for the large intestine. For example, intestinal inflammatory changes such as those associated with IBD may provide a driving force for exacerbating bacterial translocation through a disrupted epithelial layer and immune cell activation and enteric neuron dysfunction that communicates to the brain in AD. Rodent IBD studies validate that intestinal immune and functional compromise and an impaired epithelial barrier communicate to the brain to exacerbate gliosis, cytokine elevations, and increased plaque load using AD mouse models. Age, diet, environment, or disease-associated changes in the intestinal microbiome, largely localized to the large intestine, have also been characterized in human AD patients and rodent models. This dysbiosis correlates with cognitive changes and peripheral inflammatory states. Disease-modifying, unique bacteria or their components and secretory products such as LPS, small chain fatty acids, and secondary bile acids have been examined in human and rodent models to identify therapeutic targets. It is clear from rodent studies that manipulation of the intestinal microbiome is sufficient to alter brain changes such as behavior, gliosis, inflammatory changes, and Aβ levels in rodent models.