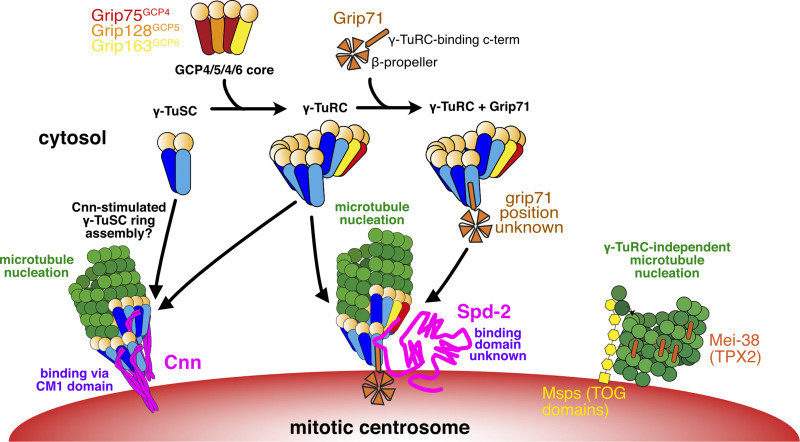
Figure 6.
Model for the different pathways of γ-tubulin complex recruitment and microtubule nucleation at mitotic centrosomes in Drosophila. This model is based on both previous data from the literature and our current findings. A mixture of γ-TuSCs and γ-TuRCs exists in the cytosol. The GCP4/5/4/6 core, predicted to comprise Grip75GCP4, Grip128GCP5, and Grip163GCP6 in Drosophila, is necessary for γ-TuSCs to assemble into γ-TuRCs within the cytosol. Grip71 is a peripheral γ-TuRC protein that can associate with cytosolic γ-TuRCs but is not necessary for their assembly and so cytosolic γ-TuRCs with and without Grip71 may exist. Cnn is able to recruit γ-tubulin in the absence, or at least near absence, of the GCP4/5/4/6 core and Grip71, suggesting it can recruit γ-TuSCs directly from the cytosol. It likely also recruits pre-formed γ-TuRCs under normal conditions because artificial Cnn scaffolds recruit Grip75GCP4-GFP (Tovey et al., 2021). Cnn’s ability to recruit γ-tubulin complexes relies on its highly conserved N-terminal CM1 domain. We speculate that CM1 domain binding may stimulate γ-TuSC oligomerization into γ-TuSC-only γ-TuRCs that could then nucleate microtubules, as is true of CM1 domain proteins in yeast. In contrast to Cnn, Spd-2 recruitment relies largely on the GCP4/5/4/6 core and so Spd-2 must predominantly recruit pre-formed γ-TuRCs. Spd-2 may be able to recruit very low levels of γ-TuSCs via Grip71 (not depicted). How Spd-2 binds to γ-tubulin complexes remains unknown. When the recruitment of γ-tubulin complexes by both Cnn and Spd-2 is inhibited, centrosomes are still able to nucleate microtubules and this γ-tubulin-independent microtubule nucleation pathway depends on Msps (the fly TOG domain protein), and possibly Mei-38 (the putative homolog of TPX2). Note that these proteins also likely facilitate γ-TuRC-dependent nucleation (not depicted).