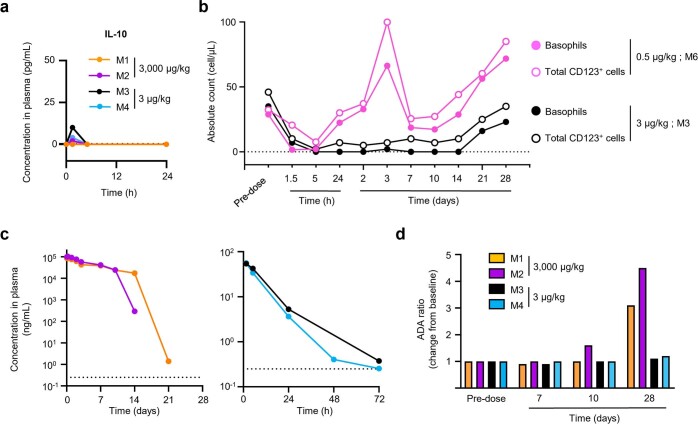
Extended Data Fig. 3. CD123-NKCE is safe and induces pharmacodynamic effects through the sustained depletion of CD123-positive cells in NHPs.
a, Cytokine production in cynomolgus monkeys treated with the high and low doses of 3 mg/kg and 3 µg/kg as single 1-hour intravenous infusion, respectively. Plasma IL-10 concentrations are shown before dosing (0), and 1.5, 5 and 24 hours after the start of the treatment. b, Numbers of circulating CD123-positive basophils (close symbols) and total CD123-positive leukocytes (open symbols) at time of study in monkeys M6 (pink) and M3 (black) treated with 0.5 and 3 µg/kg as single 1-hour intravenous infusion, respectively. c, Pharmacokinetics of the CD123-NKCE molecule in monkeys M1 (orange) and M2 (purple) treated with 3 mg/kg (left panel), and monkeys M3 (black) and M4 (blue) treated with 3 µg/kg (right panel). Plasma CD123-NKCE concentrations were monitored 1.5, 5, 24, 48, 72, 168, 240, 336, 504 and 672 hours (that is 0.04, 0.06, 0.21, 1, 2, 3, 7, 10, 14, 21 and 28 days) after the start of the one-hour infusion. The lower limit of quantification (LLOQ; 0.25 ng/mL) is indicated by the horizontal dotted line. d, Individual Anti-Drug antibody (ADA) ratio of monkeys M1 (orange) and M2 (purple) treated with 3 mg/kg, and monkeys M3 (black) and M4 (blue) treated with 3 µg/kg. Presence of ADA in plasma was monitored at predose (baseline) and at day 1, 7, 10, and 28 after the start of the one-hour infusion.