Abstract
Objective:
Overall survival is the gold-standard outcome measure for phase 3 trials, but the need for a long follow-up period can delay the translation of potentially effective treatment to clinical practice. The validity of major pathological response (MPR) as a surrogate of survival for non small cell lung cancer (NSCLC) after neoadjuvant immunotherapy remains unclear.
Methods:
Eligibility was resectable stage I–III NSCLC and delivery of PD-1/PD-L1/CTLA-4 inhibitors prior to resection; other forms/modalities of neoadjuvant and/or adjuvant therapies were allowed. Statistics utilized the Mantel–Haenszel fixed-effect or random-effect model depending on the heterogeneity (I 2).
Results:
Fifty-three trials (seven randomized, 29 prospective nonrandomized, 17 retrospective) were identified. The pooled rate of MPR was 53.8%. Compared to neoadjuvant chemotherapy, neoadjuvant chemo-immunotherapy achieved higher MPR (OR 6.19, 4.39–8.74, P<0.00001). MPR was associated with improved disease-free survival/progression-free survival/event-free survival (HR 0.28, 0.10–0.79, P=0.02) and overall survival (HR 0.80, 0.72–0.88, P<0.0001). Patients with stage III (vs I/II) and PD-L1 ≥1% (vs <1%) more likely achieved MPR (OR 1.66,1.02–2.70, P=0.04; OR 2.21,1.28–3.82, P=0.004).
Conclusions:
The findings of this meta-analysis suggest that neoadjuvant chemo-immunotherapy achieved higher MPR in NSCLC patients, and increased MPR might be associated with survival benefits treated with neoadjuvant immunotherapy. It appears that the MPR may serve as a surrogate endpoint of survival to evaluate neoadjuvant immunotherapy.
keywords: immunotherapy, major pathological response, neoadjuvant, nonsmall-cell lung cancer, survival
Introduction
Highlights
Question: Does major pathological response (MPR) after neoadjuvant Immunotherapy in resectable nonsmall-cell lung cancers predict prognosis?
Findings: Neoadjuvant immunotherapy could improve pathological responses obviously, as evidenced by a 53.4% pooled MPR rate. Neoadjuvant chemo-immunotherapy yielded promising efficacy, with an increased MPR rate compared with chemotherapy alone. MPR were shown to be associated with improved OS and disease-free survival/progression-free survival/event-free survival, and patients with stage III (vs I/II) or PD-L1 ≥1% (vs PD-L1<1%) were significantly more likely to achieve MPR.
Meaning: The findings of this meta-analysis suggest that increased MPR may associate with survival benefits in NSCLC patients treated with neoadjuvant immunotherapy. The MPR may serve as a surrogate endpoint of OS to evaluate neoadjuvant immunotherapy. Our data can be provided.
Lung cancer is one of the most common and deadly cancers in the world1. Surgical resection remains the mainstay of treatment for early-stage and locally advanced nonsmall cell lung cancer (NSCLC). However, even with early-stage disease, 30–55% of patients with NSCLC develop recurrence and die of their disease despite curative resection2–5.
A meta-analysis of the NSCLC showed that adding chemotherapy for the neoadjuvant management could get a small gain in survival of 5% at 5 years6. Immune checkpoint inhibitors (ICIs) targeting the PD‐1/PD‐L1 axis, either as monotherapy or in combination with chemotherapy, are now the cornerstone of the treatment of metastatic NSCLC. Multiple phase 2 trials of neoadjuvant immunotherapy have shown encouraging outcomes that ICI alone or combined chemotherapy, effectively reduce the size of locally advanced tumors and improve their pathological regression7. The major pathological response (MPR), defined as 10% or less viable tumor, is in the range of 19–45%with single agent in the neoadjuvant setting, and fluctuates within 33–83% when combined with chemotherapy8. Recently, neoadjuvant nivolumab plus chemotherapy showed statistically significant longer event-free survival (EFS), better pathological complete response (pCR) rate and MPR rate compared with chemotherapy alone in the phase III CheckMate-816 trial9.
Although overall survival (OS) is the gold-standard outcome measure for phase 3 trials, the need for a long follow-up period can delay the translation of potentially effective treatment to clinical practice. MPR as a candidate surrogate endpoint to rapidly evaluate the clinical efficacy of neoadjuvant chemotherapy (nCT) has also been advocated. Weissferdt et al.10 identified 151 NSCLC patients who had been treated with nCT followed by complete surgical resection from 2008 to 2012. The results revealed that MPR was associated with long-term OS on multivariable analysis (HR=2.68, P=0.01). Hellman et al.11 proposed that MPR was strongly associated with improved survival, reflected the treatment impact and captured the magnitude of the treatment benefit on survival. So far, the evidence-based validity of MPR has not been demonstrated in the immunotherapy era.
Herein, we performed a systematic review and meta-analysis to estimate the validity of MPR as a surrogate of survival after neoadjuvant immunotherapy.
Methods
Systematic review
This study was registered at the PROSPERO database. AMSTAR 2, Supplemental Digital Content 1, http://links.lww.com/JS9/A615 and PRISMA, Supplemental Digital Content 2, http://links.lww.com/JS9/A616, Supplemental Digital Content 3, http://links.lww.com/JS9/A617 were used to evaluate methodological and reporting quality12,13. A systematic literature review was performed in MEDLINE, CENTRAL, EMBASE, as well as the proceedings of the American Association for Cancer Research (AACR), the European Lung Cancer Congress (ELCC), the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO), and the International Association for the Study of Lung Cancer (IASLC) annual meetings. We additionally reviewed the reference lists of included publications along with relevant review articles retrieved from the electronic searches to identify other potentially relevant studies that could have been missed. A complete list of the search strategies for each database is provided in Supplemental Tables 1–3, Supplemental Digital Content 4, http://links.lww.com/JS9/A618.
PRISMA, Supplemental Digital Content 2, http://links.lww.com/JS9/A616 and MOOSE guidelines were followed as shown in Supplemental Tables 4–5, Supplemental Digital Content 5, http://links.lww.com/JS9/A619. The inclusion criteria of publications were defined according to the PICOD criteria, which was listed as follows. P: resectable NSCLC and confirmation of NSCLC with histopathology; I/E: neoadjuvant ICIs, including PD-1/PD-L1 inhibitors and CTLA-4 inhibitors, either combined with chemotherapy, radiotherapy, or lack thereof; C: either no control group (i.e. single-arm study); or nCT; O: MPR, disease-free survival (DFS), progression-free survival (PFS), EFS, recurrence-free survival (RFS), OS; D: randomized controlled trials, prospective nonrandomized trials, or observational (retrospective) studies. Searches did not have date restrictions and included articles in the English language that were published/presented through 12 October 2022.
More specifically, studies were eligible if they met the following inclusion criteria: histopathologically-confirmed stage I–III NSCLC with intent to perform an oncologic-quality/curative-intent resection (regardless of the proportion that eventually underwent resection); delivery of PD-1, PD-L1, or CTLA-4 inhibitors (regardless of dosing or cycles) prior to resection (nCT and/or radiotherapy, or any type of adjuvant therapy (or lack thereof), was allowed); sufficient data for quantitative meta-analysis for at least one outcome measure listed above (if the study pertained to a heterogeneous cohort, outcomes for the eligible population as defined above had to have been separately reported).
Other exclusion criteria were as follows: delivery of other treatment regimens/approaches prior to neoadjuvant therapy; meta-analyses, reviews, surveys, letters, case reports, and book chapters; studies based on the National Cancer Database or the Surveillance, Epidemiology, and End Results database, as these do not record the specific ICI agent; studies involving nonhuman subjects; and incomplete studies.
If a trial had been updated, we included only the publication with the most complete data. The data was reviewed by two independent authors (Y.J.W. and Q.L.) and validated with another two (J.W. and J.Y.C.) until consensus was reached. If important data in the included studies were missing, contacting the authors of the original publications was considered.
Data extraction
From each study, extracted data included the first author’s name, study year, study design, baseline characteristics, neoadjuvant treatment regimen(s), histology, number of patients, MPR, PFS/DFS/EFS/RFS, and OS. Of note, for purposes of this meta-analysis, PFS/DFS/EFS/RFS were used interchangeably given the similar semantics and methodologies used to calculate these outcomes.
Different articles defined MPR and PCR differently; 33 articles reported that MPR contained PCR, 7 articles reported that MPR did not contain PCR, and 13 articles did not specify whether MPR contained PCR. We added the MPR given in the article with PCR as the MPR for this study in response to these seven papers where the MPR did not include PCR. We contacted the original author by e-mail to clarify the relationship between MPR and PCR in response to these 13 articles that did not specify whether the MPR contained PCR. Of these, four articles had explicitly stated that the MPR contained PCR, and two articles’ authors had not responded to the e-mail. However, seven articles in which we were unable to determine the author’s e-mail were therefore unable to contact the author.
Evaluation of quality and bias
According to the Cochrane-Handbook for Systematic Reviews of Interventions, two authors independently assessed the methodological quality of the included randomized studies using the Cochrane Collaboration’s ‘Risk of Bias’ tool (Supplemental Figs. S1–2, Supplemental Digital Content 6, http://links.lww.com/JS9/A620, Supplemental Digital Content 7, http://links.lww.com/JS9/A621). The following domains were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment (assessed separately for self-reported and objectively assessed outcomes), incomplete outcome data, selective reporting, and other sources of bias (specifically, baseline imbalance). Each item was rated as at ‘low risk’, ‘unclear risk’, or ‘high risk’ of bias. All review authors participated in resolving any discrepancies until a consensus was reached. For nonrandomized studies, the risk of bias was assessed by the MINORS score (Supplemental Table 6, Supplemental Digital Content 8, http://links.lww.com/JS9/A622). The items were scored 0 (not reported), one (reported but inadequate), or two (reported and adequate). The global ideal score was 16 for noncomparative studies and 24 for comparative studies.
Publication bias was examined by means of constructing funnel plots. Begg’s test were conducted to analyze the publication bias of all outcomes.
Statistical analysis
The Review Manager (Rev Man) (version 5.4, provided by the Cochrane collaboration website at www.cochrane-handbook.org) software was used to evaluate publication bias, generate funnel plots and prediction intervals (PIs), evaluate for heterogeneity, as well as conduct the meta-analysis.
We assessed heterogeneity among trials using the χ 2-test for heterogeneity (with a 10% level of statistical significance) and the I 2 statistic. Data was pooled using the Mantel–Haenszel fixed-effect model if there was no significant heterogeneity (I 2<50%). If there was significant heterogeneity (I 2≥50%) the random-effect model was employed. Data regarding MPR was expressed as ORs with 95% CIs. For time-to-event data (DFS, PFS, EFS, RFS, OS), these were expressed as HRs with 95% CIs; additionally, the ORs or HRs were integrated to obtain the logHR or logOR and standard error (the inverse variance method was used to calculate the combined statistics). To quantify the association between DFS/PFS/EFS/RFS/OS and MPR, classical pairwise meta-analysis was conducted using a frequentist framework.
R software (version 4.2.0) was used to provide pooled estimates of the MPR of the single-group studies. The Meta-analysis for R (metafor) package (version 3.4-0) and the General Package for Meta-Analysis (meta) (version 5.2-0) were used to perform the random or fixed effects meta-analyses, tests for heterogeneity (I 2 and τ), generation of PIs, generation of funnel plots, and tests for publication bias (τ, which is the SD of the random-effect, to quantify study heterogeneity, was calculated using an arcsine transformation, with the value ranging from 0 to π; an inverse transform, (sin[τ/2])2, was used to express τ as a percentage in the article). The angular transformation was used and a 0.5 continuity correction was applied for studies with an event probability of 0 or 1. In addition, the restricted maximum likelihood method and the Knapp–Hartung adjustment were used. Weighted random-effect models and weighted fixed-effect models were used to determine an overall summary estimate for each outcome measure and were depicted on a forest plot with its corresponding 95% CI and associated 95% PIs. PIs were included because they were particularly insightful in this setting, with a 95% PI providing a prediction region for a single future study. The R code used to generate each of these analyses is provided in the Supplement 3, Supplemental Digital Content 9, http://links.lww.com/JS9/A623.
A sensitivity analysis was performed using Stata v12.0 according to the ‘leave-one-out’ method, which was used to determine the impact of each individual study on the overall results by removing each study. Each estimate value and its upper and lower CIs represented the HR or OR after the individual study was removed. The critical value of OR was 1 and the critical value of HR was 0. After removing any individual study, if the new HR or OR value was consistent with the original HR or OR value on the same side of the critical value, it was considered to verify the robustness of the particular outcome parameter.
Results
Literature review
Fifty-three articles met the criteria for this meta-analysis (Fig. 1), which included 7 randomized trials, 29 prospective nonrandomized trials, and 17 retrospective studies. There were nine studies that compared neoadjuvant chemo-immunotherapy (nCIT) versus nCT alone. Table 1 displayed pertinent details of each study9,14–65; patients were most commonly stage III and received 2–4 cycles of a variety of ICIs.
Figure 1.
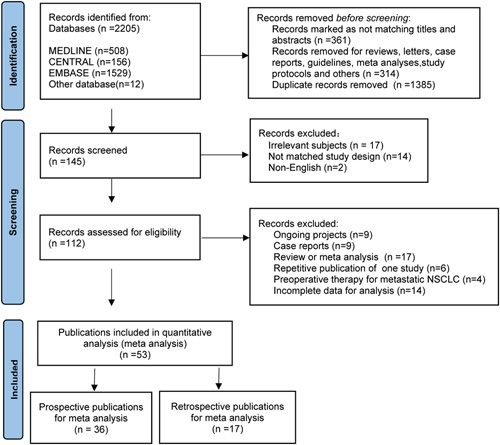
PRISMA flowchart of study selection.
Table 1.
General information from the included studies.
| OS | EFS/DFS/PFS | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Trial type | No. of patients,n | Resectable patients, %(n) | Histology | Clinical stage | R0% (n) | MPR% (n) | ORR% (n) | DCR% (n) | pCR% (n) | Survival (median/%) | Survival (median/%) | Neoadjuvant regimen | Neoadjuvant cycle | Adjuvant IO | ≥G3 TRAEs%(n) | ≥G3 SRAEs%(n) | Fatal AEs(n) |
| Forde PM et al, 2022 9 | randomised, controlled, phase III | 179 | 149 (83.2%) | NSCLC | IB-IIIA | 83.2% (124/149) | 36.9% (66/179) | 53.6% (96/179) | 92.7% (166/179) | 24% (43/179) | NR (median) | 31.6 months (median),76.1% (1-year)63.8% (2-year) | nivolumab+Chemo | 3 | -- | 33.5% | -- | 5 |
| 179 | 135 (75.4%) | 77.8% (105/135) | 8.9% (16/179) | 37.4% (67/179) | 86.6% (155/179) | 2.2% (4/179) | NR (median) | 20.8 months (median) 63.4% (1-year)45.3% (2-year) | Chemo | -- | 36.9% | -- | 4 | |||||
| Cascone, T. et al, 2021 14 | randomized, phase II | 23 | 22 (95.7%) | SCC(39%), ASCC(2%), AC(59%) | I-IIIA | 100% (22/22) | 22% (5/23) | 22% (5/23) | 87% (20/23) | 9% (2/22) | NR (median) | NR (median) | nivolumab | 3 | -- | 13%(3/23) | -- | 1 |
| 21 | 17 (81.0%) | 100% (17/17) | 38% (8/21) | 19% (4/21) | 81% (17/21) | 29% (6/21) | NR (median) | NR (median) | nivolumab+ipilimumab | 3 | -- | 10%(2/21) | -- | 0 | ||||
| Provencio et al.2022 15 | randomized, phase II | 57 | 53 (93.0%) | NSCLC | IIIA-B | 92.5%49/53 | 52% (30/57) | 74% (42/57) | – | 36.2% (21/57) | 84.7% (2-year) | 66.6% (2-year) | Nivolumab+Chemo | 3 | 6 mo | 24.6%(14/57) | 0 | |
| 29 | 20 (69.0%) | 65.0% (13/20) | 14% (4/29) | 48% (14/29) | 6.8% (2/29) | 63.4% (2-year) | 42.3% (2-year) | Chemo | 3 | 10.3%(3/29) | ||||||||
| Feng, Y et al, 202116 | randomized, phase II | 8 | 8 (100.0%) | SCC(90.5%),Non-SCC(9.5%) | IIA-IIIB | – | 50% (4/8) | 87.5% (7/8) | 100% (8/8 | 37.5% (3/8) | – | – | pembrolizumab or toripalimab+Chemo | 2 | -- | 12.5%(1/8) | -- | 0 |
| 13 | 13 (100.0%) | – | 38.46% (5/13) | 46.15% (6/13) | 100% (13/13) | 7.69% (1/13) | – | – | Chemo | 2 | -- | 0 | -- | 0 | ||||
| Lei J et al, 2020 17 | randomised, controlled, phase II trial | 14 | 7 (50%) | -- | IIIA-IIIB | – | 28.6% (2/7) | 86.7% (6/7) | – | 57.1% (4/7) | – | – | camrelizumab+Chemo | 3 | -- | -- | -- | 0 |
| 13 | 6 (46.2%) | – | 16.7% (1/6) | 57.1% (4/6) | – | 16.7% (1/6) | – | – | Chemo | 3 | -- | -- | -- | 0 | ||||
| Altorki, N. K. et al, 2021 18 | randomized, phase II | 30 | 26 (86.7%) | AC(53%),SCC(37%),Sarcomatoid(3%), NOS(7%) | I-IIIA | 88% (23/26) | 6.7% (2/30) | 3% (1/30) | 90% (27/30) | 0 | – | – | durvalumab | 2 | 12 cycles | 20%(6/30) | -- | 1 |
| 30 | 26 (86.7%) | 96% (25/26) | 53.3% (16/30) | 47% (14/30) | 96.7% (29/30) | 26.6% (8/30) | – | – | durvalumab+RT | 2 | 12 cycles | 23.3%(7/30) | -- | 1 | ||||
| Qiu FM et al,202219 | Randomized, phase II | 60 | -- | NSCLC | IB-IIIA | 91.7% (55/60) | 41.4% (12/29) | 55.2% (16/29) | – | 24.1% (7/29) | – | – | Two cycles sintilimab+Chemo | 1 y | 5%(3/60) | -- | -- | |
| 26.9% (7/26) | 50% (13/26) | – | 19.2% (5/26) | – | – | Three cycles sintilimab+Chemo | -- | -- | ||||||||||
| Hou X et al,2022 20 | Prospective, observational study | 31 | 31 (100.0%) | SCC(48.3%)AC(45.2%)Others(6.5%) | IIIA-IIIB | – | 61.3% (19/31) | 64.5% (20/31) | 100% (31/31) | 25.8% (8/31) | 95.0% (1-year) | 91.6% (1-year) | camrelizumab+ Chemo | 3 | ≥2 | -- | -- | -- |
| 25 | 24 (96.0%) | SCC(60.0%)AC(40.0%) | – | 37.5% (9/24) | 40.0% (10/25) | 92.0% (23/25) | 8.3% (2/24) | 83.2% (1-year) | 57.0% (1-year) | Chemo | 3 | ≥2 | -- | -- | -- | |||
| Liang, H et al, 2021 21 | retrospective | 10 | 10 (100.0%) | SCC(60%),AC(20%),large-cell(5%),Others(15%) | IIB-IIIB | – | 50% (5/10) | 80% (8/10) | 100% (10/10) | 10% (1/10) | 100% (10/10) | 100% (10/10) | PD-1 inhibitors+Chemo | 1-6 | -- | 0 | 0 | 0 |
| 10 | 10 (100.0%) | – | 30% (3/10) | 30% (3/10) | 80% (8/10) | 0 | 60% (6/10) | 60% (6/10) | Chemo | 1-6 | -- | -- | -- | 0 | ||||
| Liu Z et al, 2021 22 | retrospective | 79 | 79 (100.0%) | SCC(55.9%),AC(32.4%),ASCC(2.9%),large-cell(5.9%),sarcomatoid(1.2%),others (1.8%) | IB-IIIB | 100% (79 /79) | 53.2% (42/79) | 70.9% (56/79) | 98.7% (78/79) | – | – | 13.28 months (median), 67.2% (2-year) | pembrolizumab/nivolumab/sintilimab/camrelizumab+Chemo | 3 (2-5) | -- | -- | -- | -- |
| 91 | 91 (100.0%) | 100% (91 /91) | 14.3% (13/91) | 47.3% (43/91) | 95.7% (87/91) | – | – | 12.6 months (median),39.5% (2-year) | Chemo | 2 (2-5) | -- | -- | -- | -- | ||||
| Zhao D et al, 202223 | retrospective | 42 | 42 (100.0%) | SCC(69.0%)AC(19.0%)Others(11.9%) | IB-IIIB | 100% (42/42) | 71.4% (30/42) | 59.5% (25/42) | 80.9% (34/42) | 40.5% (17/42) | – | – | pembrolizumab + Chemo | 2-4 | -- | -- | -- | 1 |
| 98 | 98 (100.0%) | SCC(54.1%)AC(37.8%)Others(8.2%) | 100% (98/98) | 14.3% (14/98) | 22.4% (22/98) | 71.4% (70/98) | 6.1% (6/98) | – | – | Chemo | 2-4 | -- | -- | -- | 3 | |||
| Huang Z et al, 2021 24 | Retrospective | 25 | 24 (96.0%) | AC(66.3%), SCC(24.8%), Others(8.9%) | IIIA | 95.8% (23/24) | 37.5% (9/24) | 32.0% (8/25) | 96.0% (24/25) | 4.2% (1/24) | – | – | nivolumab | 2 | -- | 12%(3/25) | 12.5%(3/24) | 0 |
| 82 | 78 (95.1%) | 84.6% (66/78) | 12.8% (10/78) | 53.7% (44/82) | 95.1% (78/82) | 2.6% (2/78) | – | – | Chemo | 2 | -- | 20.7%(17/82) | 16.7%(13/78) | 0 | ||||
| Chaft J E et al, 202225 | prospective, single-arm | 181 | 159 (88%) | SCC(38%), Others(62%) | IB-IIIB | 86.2% (137/159) | 20.3% (29/143) | 6.1% (11/181) | 87.3% (158/181) | 5.6% (8/143) | – | – | atezolizumab | 1-2 | -- | 11.0%(20/181) | -- | -- |
| Zhang Y. et al, 202226 | prospective, single-arm | 26 | 17 (65.4%) | SCC | IIB-IIIB | – | 38.5%(10/26) | – | – | 19.2% (5/26) | – | – | Camrelizumab+Chemo | 2-4 | -- | 7.6%(2/26) | -- | -- |
| Bahce I. et al, 202227 | prospective, single-arm | 26 | 24 (92.3%) | NSCLC | IIB-IIIB | – | 79.2% (1924) | – | – | 62.5% (15/24) | – | – | Ipilimumab+nivolumab+Chemo+RT | -- | -- | 54%(14/26) | ||
| Gao Y et al, 202228 | prospective, single-arm | 44 | 44 (100.0%) | NSCLC | III | 100.0% (44/44) | 84.1% (37/44) | – | – | 59.1% (26/44) | – | – | PD-1 / PD-L1 inhibitors+Chemo | 3 | -- | 18.2%(8/44) | -- | -- |
| Lin YB et al,2022 29 | prospective, single-arm | 37 | 27 (73.0%) | SCC(78.4%) Others(21.6%) | IIB-III | 96.0% (26/27) | 81.5% (22/27) | – | – | 48.1% (13/27) | – | – | tislelizumab+ Chemo | 3-4 | -- | 2.7%(1/37) | -- | -- |
| Yan S et al,2022 30 | prospective, single-arm | 53 | 39 (73.6%) | SCC(79.2%) Others(20.8%) | IIB-IIIB | 100.0% (39/39) | 61.4% (25/39) | 85.7% (42/49) | – | 51.3% (20/39) | – | – | tislelizumab+ Chemo | 2-4 | -- | 30.6%(15/49) | -- | -- |
| Zhang Y et al,2022 31 | prospective, single-arm | 40 | 26 (65.0%) | SCC(87.9%) Others(12.1%) | IIIA-IIIB | 100.0% (26/26) | 57.7% (15/26) | – | – | 42.3% (11/26) | – | 89.4% (1-year)72.9% (2-year) | toripalimab+ Chemo | 2-4 | 2 cycles+13 cycles | -- | -- | -- |
| Wang J et al.2021 32 | prospective, single-arm | 72 | 72 (100.0%) | SCC(1.4%),AC(6.9%),SCLC(91.7%) | IIIA | – | – | 94.4% (68/72) | 98.6% (71/72) | 29.1% (21/72) | – | – | PD-1 inhibitors+Chemo | 2 | -- | 19.4%(14/72) | -- | 0 |
| Duan H et al.2021 33 | prospective, single-arm | 23 | 20 (87.0%) | AC(17.4%), SCC(82.6%) | IIA-IIIB | 95% (19/20) | 50% (10/20) | 73.9% (17/23) | 100% (23/23) | 30% (6/20) | – | 11.3 months (median) | PD-1 inhibitors+Chemo | 1-4 | -- | 30%(6/20) | -- | -- |
| Zhang, Y. et al, 202134 | retrospective | 56 | 45 (80.4%) | NSCLC | IIIA-IIIB | 100% (45/45) | 68.9% (31/45) | – | – | 40% (18/45) | – | – | pembrolizumab/toripalimab+Chemo | -- | -- | 5.4%(3/56) | -- | -- |
| Zinner, R. et al, 202035 | prospective, single-arm | 13 | 13 (100.0%) | SCC(69%),non-SCC(31%) | -IIIA | – | 46% (6/13) | 46% (6/13) | – | 38% (5/13) | – | – | nivolumab+Chemo | 3 | -- | 30.8%(4/13) | -- | -- |
| Provencio et al.2020 36 | prospective, single-arm, phase II | 46 | 41 (89.1%) | SCC(35%),AC(57%),NOS(9%) | IIIA | 100% (41/41) | 83% (34/41) | 76.1% (35/46) | 100% (46/46) | 63% (26/41) | 97.8% (1-year)93.5% (18 months)89.9% (2-year) | 95.7% (1-year)87% (18 months)77.1% (2-year) | nivolumab+Chemo | 3 | -- | 30%(14/46) | -- | -- |
| Forde PM et al.2018 37 | prospective, single-arm | 22 | 21 (95.5%) | AC(62%),SCC(29%),others(10%) | I-IIIA | 95% (20/21) | 45% (9/20) | 9.5% (2/21) | 95.2% (20/21) | 15% (3/20) | – | RFS:73.0% (18 months) | nivolumab | 1 | -- | 4.5%(1/22) | -- | -- |
| Bott MJ et al.2018 38 | prospective, single-arm, phase I | 22 | 20 (90.9%) | AC(67%),SCC(24%),ASCC(5%), Pleomorphic(5%) | I-IIIA | – | 45% (9/20) | 9.5% (2/21) | 95.2% (20/21) | – | – | – | nivolumab | 2 | -- | 5%(1/20) | -- | -- |
| Shen D et al, 2021 39 | prospective, single-arm | 37 | 37 (100.0%) | SCC(100%) | IIB-IIIB | 100% (37/37) | 64.9% (24/37) | 86.5% (32/37) | 100% (37/37) | 45.9% (17/37) | – | – | pembrolizumab+Chemo | 2 | -- | -- | -- | -- |
| Eichhorn F et al, 2021 40 | prospective, single-arm, phase II | 15 | 15 (100.0%) | AC(86.7%), SCC(13.3%) | II-IIIA | 100% (15/15) | 13.3% (2/15) | 26.7% (4/15) | 93.3% (14/15) | 13.3% (2/15) | – | – | pembrolizumab | 2 | -- | 20%(3/15) | -- | -- |
| Bar, J., et al, 202141 | prospective, single-arm | 26 | 23 (88.5%) | AC(50%),SCC(42%),ASCC(4%),NSCLC(4%) | I-II | – | 27% (7/26) | 4% (1/26) | 84.6% (22/26) | 12% (3/26) | – | – | pembrolizumab | -- | -- | 8%(2/26) | -- | -- |
| Tong BC et al, 2021 42 | prospective, single-arm, phase II trial | 30 | 25 (83.3%) | AC(33%),SCC(57%),others(10%) | IB-IIIA | 88% (22/25) | 28% (7/25) | – | – | 12% (3/25) | – | – | pembrolizumab | 2 | 4 cycles | 3.3%(1/30) | -- | -- |
| Shu CA et al, 2020 43 | prospective, single-arm, phase II | 30 | 29 (96.7%) | SCC(40%),AC(57%),Large cell neuroendocrine(3%) | IB-IIIA | 87% (26/30) | 57% (17/30) | 63.3% (19/30) | 93.3% (28/30) | 33% (10/30) | – | 17.9 months (median) | atezolizumab+Chemo | 2-4 | -- | -- | -- | 3 |
| Lee J et al, 2021 44 | prospective, single-arm, phase II | 181 | 159 (87.8%) | SCC(38%),NSCC(62%) | IB-IIIB | 91% (145/159) | 20% (30/147) | – | – | 7% (10/147) | – | – | atezolizumab | 2 | -- | 9(5.0%) | 20(12.6%) | 1 |
| Zhang P et al, 202245 | prospective, single-arm, phase II trial | 50 | 30 (60.0%) | AC(22%),SCC(56%), NOS(22%) | IIIA | 100% (30 /30) | 43.3% (13/30) | 46% (23/50) | 96% (48/50) | 20% (6/30) | 93.7% (1-year) | 85.3% (1-year) | sintilimab+Chemo | 2-4 | -- | 8%(4/50) | -- | 1 |
| Gao S et al, 2020 46 | prospective, single-arm, phase Ib | 40 | 37 (92.5%) | SCC(82.5%),AC(15%),Mixed(2.5%) | IA-IIIB | 97.3% (36/37) | 40.5% (15/37) | 20% (8/40) | 90% (36/40) | 16.2% (6/37) | – | – | sintilimab | 2 | -- | 10%(4/40) | -- | 1 |
| Tao XL et al, 2020 47 | prospective, single-arm, phase Ib | 36 | 36 (100.0%) | AC(16.7%),SCC(80.6%),Mixed(2.8%) | IA-IIIB | – | 36.1% (13/36) | 36.1% (13/36) | 94.4% (34/36) | 13.9% (5/36) | – | – | sintilimab | 2 | -- | -- | -- | -- |
| Zhu, X et al, 202148 | prospective, single-arm | 48 | 22 (45.8%) | SCC(64.6%), AC(18.8%),NSCLC(16.6%) | IIA-IIIC | 100% (22/22) | 40.9% (9/22) | 62.5% (30/48) | 79.2% (38/48) | 18.2% (4/22) | – | – | toripalimab+Chemo | 2-4 | -- | 6.2%(3/48) | -- | -- |
| Wu YL et al, 2022 49 | prospective, single-arm, phase 1b/3 | 37 | 34 (91.9%) | AC(13.5%), SCC(83.8%),ASCC(2.7) | II-IIIB | 94.1% (32/34) | 55.9% (19/34) | 70.3% (26/37) | 97.3% (36/37) | 32.4% (11/34) | – | – | PD-1 inhibitors+Chemo | 3 | 16 cycles | 78.4%(29/37) | 17.6%(6/34) | |
| Zhao ZR et al, 2021 50 | prospective, single-arm, phase II | 33 | 30 (90.9%) | NSCLC | IIIA-IIIB | 96.7% (29/30) | 66.7% (20/30) | 87.9% (29/33) | 97% (32/33) | 50% (15/30) | – | – | toripalimab+Chemo | 3 | 12 months | 6%(18.2) | -- | -- |
| Hong MH et al, 2021 51 | prospective, single-arm, phase II | 14 | 11 (78.6%) | AC(50%),others(50%) | III | 100% (11/11) | 72.7% (8/11) | – | – | 27.3% (3/11) | – | – | durvalumab+Chemo+RT | 2 | 12 months | 7%(1/14) | -- | -- |
| Rothschild SI et al, 202152 | prospective, single-arm, phase II trial | 67 | 55 (82.1%) | SCC(33%),AC(55%),large-cell neuroendocrine carcinoma(2%),NOS(10%) | IIIA | 93% (51/55) | 62% (34 /55) | 58% (36 /62) | 100% (55/55) | 18% (10/55) | 91% (1-year)83% (2-year) | EFS: 73% (1-year) | durvalumab+Chemo | 3 | 26 cycles | 88%(59 /67) | 87%(48 /55) | 2 |
| Tfayli A et al, 2020 53 | prospective, single-arm, phase II | 15 | 11 (73.3%) | SCC(13.3%), AC(86.7%) | IB-IIIA | – | 9.1% (1/11) | 27.2% (3/11) | 81.8% (9/11) | 6.7% (1/15) | – | – | avelumab+Chemo | 3 | -- | 26.7%(4/15) | -- | -- |
| Deng H et al, 2021 54 | retrospective | 51 | 31 (60.8%) | SCC(77.4%), AC(19.4%), ASCC(3.2%), large cell lung cancer(3.2%), lymphoepithelioma-like carcinoma(6.5%) | IIIB | 96.8% (30 /31) | 67.8% (21/31) | 71% (22/31) | 100% (31/31) | 35.5% (11/31) | – | 27.5 months (median) | sintilimab/pembrolizumab/camrelizumab/nivolumab/tislelizumab+Chemo | 3.4 | -- | -- | -- | -- |
| Shi, L., et al, 202155 | retrospective | 27 | 27 (100.0%) | SCC | IIA-IIIB | – | 55.6% (15/27) | – | – | 29.6% (8/27) | – | – | sintilimab,/pembrolizum/camrelizumab/toripalimab/tislelizumab+Chemo | 1-4 | -- | 66.6%(18/27) | -- | -- |
| Hong T et al, 2021 56 | retrospective | 25 | 25 (100.0%) | SCC(76%), AC(20%), Others(4%) | IIA-IIIC | 100% (25/25) | 52% (13/25) | 88% (22/25) | 100% (25/25) | 32% (8/25) | – | – | sintilimab/pembrolizumab/camrelizumab+Chemo | 3 | -- | -- | -- | -- |
| Hu Y et al, 202157 | retrospective | 20 | 20 (100.0%) | AC(20%), SCC(70%), ASCC(5%), large-cell neuroendocrine carcinoma(5%) | IB-IIIB | 100% (20/20) | 40% (8/20) | 75% (15/20) | 100% (20/20) | 25% (5/20) | – | – | sintilimab/pembrolizumab/tislelizumab/toripalimab++Chemo | 2-4 | -- | 0 | -- | -- |
| Cheng X. et al, 202258 | retrospective | 19 | 19 (100%) | SCC(63.2%),Other(36.8%) | IIIA-IIIB | 100% (19/19) | 79.0% (15/19) | – | – | 52.6% (10/19) | – | – | Tislelizumab+Chemo | 2-4 | -- | -- | -- | -- |
| Chen T et al, 2021 59 | retrospective | 12 | 12 (100.0%) | SCC(33.3%), AC(50.0%), ASCC(8.3%)Undifferentiated adenocarcinoma (8.3%) | IIIA-IIIB | 100% (12/12) | 33.3% (4/12) | 50% (6/12) | 100% (12/12) | 41.7% (5/12) | – | – | pembrolizumab/nivolumab+Chemo | -- | -- | -- | -- | |
| Wu J et al, 202260 | retrospective | 76 | 76 (100.0%) | AC(22%), SCC(65%), NSCLC-NOS(13%) | IB-IIIB | 100% (76/76) | 64% (49/76) | 75% (57/76) | 100% (76/76) | 37% (28/76) | – | – | pembrolizumab/nivolumab+Chemo | 2-4 | -- | 25%(19 /76) | -- | -- |
| Zhang, Y et al, 202161 | retrospective | 30 | 23 (76.7%) | SCC(73.3%), others(26.7%) | IIIA-IIIB | 100% (23/23) | 69.6% (16/23) | – | – | 30.4% (7/23) | – | – | pembrolizumab/toripalimab+Chemo | 2 | -- | 10%(3/30) | -- | -- |
| Chen Y et al, 2021 62 | Retrospective, single-arm | 35 | 35 (100.0%) | SCC(74.3%), AC(20%), Large-cell carcinoma(2.9%),Sarcomatoid carcinoma(2.9%) | IIIA-IIIB | 100% (35/35) | 74.3% (26/35) | 48.6% (17/35) | 100% (35/35) | 51.4% (18/35) | – | – | pembrolizumab+Chemo | 2 | -- | 2.9%(1/35) | -- | -- |
| Zhai H et al, 2022 63 | retrospective | 46 | 45 (97.8%) | AC(41.3%), SCC(58.7%) | IIIA-IIIB | 95.6% (43/45) | 17.8% (8/45) | 60.9% (28/46) | 97.8% (45/46) | 53.3% (24 /45) | 90.5% (1-year), 86.8% (18 months)79.9% (2-year) | 67% (1-year), 53.4% (18 montyhs)45.8% (2-year) | nivolumab+Chemo | 3 | at least 1 cycle | 19.6%(9/46) | -- | -- |
| Yao Y et al, 2022 64 | retrospective | 11 | 11 (100%) | AC(8.9%), SCC(91.1%) | IIIA-IIIB | 100% (11/11) | 81.8% (9/11) | 72.7% (8/11) | 100% (11/11) | 72.7% (8/11) | – | – | camrelizumab/ durvalumab+Chemo | 2-3 | 1-2 cycles | -- | -- | -- |
| Fan BS et al,2022 65 | retrospective | 8 | 8 (100.0%) | SCC(75.0%)AC(25.0%) | IIIA/IIIB | 100.0% (8/8) | 62.5% (5/8) | 87.5% (7/8) | 100% (8/8) | – | – | – | sintilimab+Chemo | 1-3 | -- | 12.5%(1/8) | -- | -- |
Therefore they were included in our meta-analysis due to different purposes.
AC, adenocarcinoma; AEs, adverse events; ASCC, adenocarcinoma squamous cell cancer, Chemo:Chemotherapy; DCR, disease control rate; DFS, disease-free survival; EFS, event-free survival; G3, grade 3; MPR, major pathological response; NOS, Not otherwise specified; NR, not reached; NSCLC, non small cell lung cancer; ORR, objective response rate; OS, overall survival; pCR, pathological complete response; PFS, progression-free survival; R0, R0 resection (no residual tumor); RFS, recurrence-free survival; RT, Radiotherapy; SCC, squamous cell cancer; SCLC, small cell lung cancer; SRAE, surgery-related adverse events; TRAE, treatment-related adverse events.
MPR of neoadjuvant immunotherapy
Fifty-three articles were included. Of note, Cascone et al.14 and Altorki et al.18 reported MPR rates of neoadjuvant ICI alone and ICI combination regimens, and Qiu19 reported MPR rates of neoadjuvant ICI 2 cycles and 3 cycles. For these three articles, each arm needed to be analyzed as an independent data. After pooled analysis of the 56 MPR data, the estimated rate was 53.8% (95% CI 47.0–59.6%, 95% PI 16.9–88.4%; I 2=88% 95% CI 85–90%; τ=0.20, 95% CI 0.16–0.25) (Fig. 2).
Figure 2.
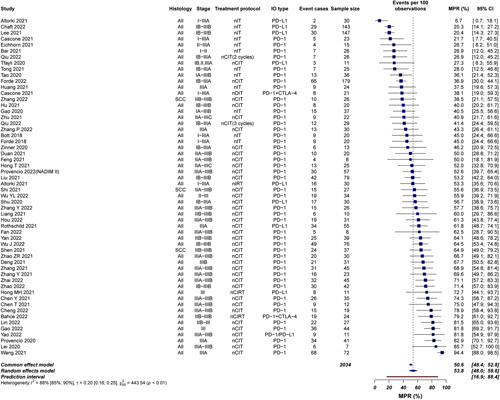
Weighted random-effects model of major pathological response (MPR). Of note, Cascone et al.14 and Altorki et al.18 reported MPR rates of neoadjuvant ICI alone and ICI combination regimens, and Qiu 19 reported MPR rates of neoadjuvant ICI 2 cycles and 3 cycles; therefore, for all these studies, data of each were analyzed as two groups.
Comparison MPR of nCIT vs nCT alone
There were eight comparative studies from the available data; of these, the most common comparison was nCIT versus nCT alone (n=8; five prospective and three retrospective).
As compared to nCT, patients who underwent nCIT were associated with higher MPR rates; the OR was 6.19 (95% CI 4.39–8.74, P<0.00001) (Fig. 3). There was no significant heterogeneity.
Figure 3.
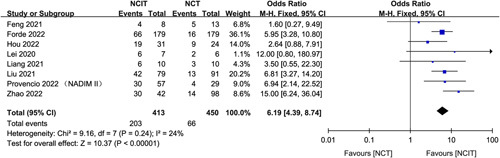
Pooled analyses of MPR comparing between nCIT and standard nCT.
Correlation between MPR and survival outcome
We explored whether MPR could be an indicator for survival. As a result, HR for DFS/PFS/EFS and OS by MPR status crossed six studies and four studies, respectively. The overall HR for DFS/PFS/EFS was 0.28 (95% CI: 0.10–0.79, P=0.02), indicating a statistically significant association between MPR and DFS/PFS/EFS (Fig. 4). The overall HR for OS was 0.80 (95% CI: 0.72–0.88, P<0.0001), indicating a statistically significant association between MPR and OS (Fig. 4).
Figure 4.
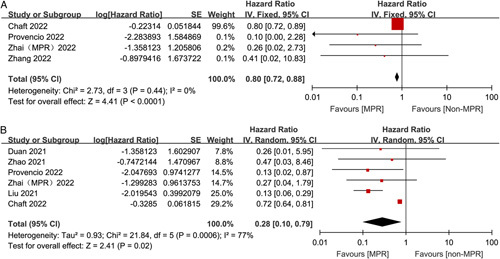
Association between MPR and time-to-event outcomes.
Correlation between MPR and ORR
Although a minority of studies reported the relationship between MPR and ORR (n=17), the results were statistically significant and all without heterogeneity. MPR was associated with a OR of 6.21 for ORR (95% CI 3.71–9.16, P<0.00001) (Fig. 5).
Figure 5.
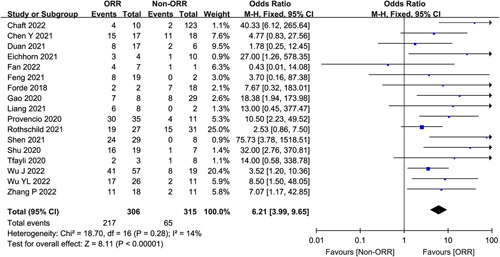
Association between MPR and ORR.
Predictors of MPR and association with outcomes
Lastly, we examined several potential predictors of developing MPR with neoadjuvant immunotherapy (with or without other neoadjuvant therapies), such as PD-L1 expression, histology, and clinical stage. Ten studies reported MPR rates by PD-L1 tumor proportion score. Patients with PD-L1 ≥1% were significantly more likely to achieve MPR (OR 2.21, 95% CI 1.28–3.82, P=0.004, Fig. 6) than that with PD-L1 negative (<1%). Eight studies reported MPR rates by clinical stage (III vs I/II). Patients with stage III were significantly more likely to achieve MPR (OR 1.66, 95% CI 1.02–2.70, P=0.04, Fig. 6) than stage I/II patients. Histology (squamous cell vs nonsquamous) was not significantly associated with MPR rates (Fig. 6). There was no heterogeneity in any of the aforementioned parameter.
Figure 6.
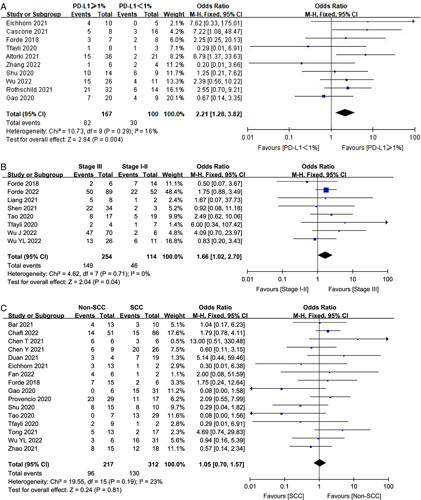
Major pathological response rates by PD-L1 expression, clinical stage and histology.
Sensitivity analysis and statistical analysis of publication bias
Sensitivity analysis by systematically eliminating each specific study from the total count demonstrated that the new HRs or ORs were similar to the original HRs/ORs as above (Supplemental Table 7, Supplemental Digital Content 10, http://links.lww.com/JS9/A624), implying that any given study might not have disproportionately impacted the results. Additionally, statistical analysis of publication bias demonstrated no statistically significant evidence thereof (Supplemental Figure S3, Supplemental Digital Content 11, http://links.lww.com/JS9/A625).
Discussion
Neoadjuvant therapy has the potential to improve the survival of resectable NSCLC patients. The challenge remains to determine the best neoadjuvant approach to achieve a high response rate and acceptable toxicity. Immunotherapy recently emerges as a promising therapeutic strategy for NSCLC, and some focus has shifted to the use of ICI in early-stage NSCLC. The delivery of early ICI therapy may lead to a deep pathological response because of the antigen load of the entire cancer prior to surgical resection66,67. Brandt et al.68 reported that the MPR rate following nCT was 15%. The CheckMate 159 research showed that neoadjuvant nivolumab achieved MPR in 45% of participants37. The phase II NADIM study evaluated the effectiveness of nivolumab combined with carboplatin/paclitaxel as neoadjuvant therapy in patients with stage IIIa resectable NSCLC. A high MPR rate of 82.9% suggested that nCIT might be a new option for patients with locally advanced NSCLC36. It was determined that nivolumab plus chemotherapy showed statistically significant improvement compared to neoadjuvant platinum-based chemotherapy alone in the MPR as a neoadjuvant treatment for resectable NSCLC in the phase III CheckMate-816 trial by 36.9 versus 8.9%, respectively9. In our meta-analysis, the pooled MPR was 53.8%, which demonstrated the addition of immunotherapy to nCT could improve pathological responses obviously. Our data confirmed that nCIT yielded promising efficacy, with an increased MPR rate compared with chemotherapy alone (49.2 vs 14.7%). The synergistic effect of chemotherapy and ICIs, with the cytotoxic chemotherapy increasing the recognition of these agents as immunotherapies, might explain the high rates of MPR69,70.
The median time from trial initiation to publication for adjuvant platinum chemotherapy trials in NSCLC was longer than 10 years11. Early and widespread acceptance of new perioperative treatment strategies for resectable NSCLC was usually hindered by a lack of surrogate endpoints of clinical efficacy. Pathological response has shown patient-level association with survival in various cancers71–73, which requires further evaluation across ongoing trials of neoadjuvant therapy involving patients with NSCLC.A meta-analysis based on 21 clinical studies showed that the HR of OS under different pCR states was 0.49 (95% CI 0.43–0.56)74. Another combined analysis of two nCT studies showed that, 5-year OS was 80.0% in the pCR group versus 55.8% in the non-pCR group (P=0.0007), and pCR was a favorable prognostic factor of OS (HR 0.34; 95% CI 0.18–0.64)72. Maybe the low pCR rate after neoadjuvant treatments and insufficient data were available for analysis, its use was greatly limited as a surrogate endpoint. Compared with pCR, MPR is seemed more common. Although without mediastinal downstaging evaluation, MPR has been accepted as another surrogate of survival in patients with NSCLC received nCT. William et al.74 reported the MPR rate following nCT was 30% and histopathologic response was a significant predictors of OS. The College of American Pathologists recommended MPR as one of the study endpoint of clinical trials on neoadjuvant immunotherapy for lung cancer. However, the relationship between OS and MPR in resectable NSCLC patients who receiving neoadjuvant immunotherapy has not been fully elucidated. The present meta-analysis synthesized the results of published clinical trials, we found that MPR were shown to be associated with improved OS (HR=0.80, 95% CI: 0.72–0.88, P<0.0001) and DFS/PFS/EFS (HR=0.28, 95% CI: 0.10–0.79, P=0.02) when compared with non-MPR. MPR seemed to be an alternative endpoint of OS in patients with NSCLC received nCT.
In the MRC LU22/NVALT 2/EORTC 08012 multicenter randomised trial, nCT resulted in a good radiological response rate (4% CR, 45% PR). However, there was no evidence of a benefit in terms of OS. The discrepancy between the radiographic and pathological assessment was often observed. The tumor response patterns of Immune agents may differ compared with conventional chemotherapeutic agents75. The incidence of radiographic partial response and complete response with nCIT ranged from 38 to 72%7,36,43,52,77. Pseudo progression, which was characterized by radiologic progression of the tumor burden, followed by objective response, was first described in melanoma patients treated with ipilimumab78. Some studies indicated that pseudo progression may occur in other cancer types receiving ICI therapy. This unconventional phenomenon does not typically occur with traditional cytotoxic chemotherapy. In the present study, objective response with neoadjuvant immonotherapy indicated an increased likelihood of MPR (OR=6.21, 95% CI 3.71–9.16, P<0.00001). Although new immunotherapy-specifc radiologic criteria has been developed, the classic RECIST remains a reasonable and meaningful method to assess response to immunotherapy in the clinic79.
Recent trials had evaluated potential predictive biomarkers for MPR, but there was no consensus currently. Two dominant subtypes, accounting for ~80% of NSCLC cases, are lung adenocarcinoma and lung squamous carcinoma (SCC)80. Several studies have reported relatively higher MPR rates in SCC patients, compared with adenocarcinoma43,81. In our pooled analysis, there was no difference (44.2 vs 41.7%, P=0.81) in MRP rates between SCC and non-SCC.
Usually, patient with earlier-stage disease (stages IB to II) are recommended for upfront resection and adjuvant chemotherapy based on a series of large prospective trials and the Lung Adjuvant Cisplatin Evaluation meta-analysis82. It is still unclear as to which stages of NSCLC benefit the most from neoadjuvant ICI therapy. A stage-based assessment of pathological responses is important as it may allow for improved design of future trials in specific disease stages69. As reported in the CheckMate-816 trial (NCT02998528), the magnitude of EFS benefit was greater for the patients with stage IIIA (HR 0.54) than for those with stages IB to II disease (HR 0.87) and for patients with tumor PD-L1 expression of 1% or greater (HR 0.41) than for those with PD-L1 expression lower than 1% (HR 0.85). The MPR benefit for stage IIIA patients with the addition of nivolumab in CheckMate-816 was more impressive than that for stage IB to stage II9. In our meta-analysis, eight studies explored MPR rates of different stages, and similar to the previous perioperative chemotherapy, patients with stage III were significantly more likely to achieve MPR (OR=1.66, 95% CI 1.02–2.70, P=0.04).
Irrespective of the results in metastatic stage IV patients, the predictive value of the PD-L1 status might have a different impact in patients with non-metastatic earlier-stage lung cancer with less tumor burden40. Both the NEOSTAR14 and the Checkmate-816 trial9 showed that elevated PD-L1 expression was also associated with higher pathologic responses. However, both the CLMC3 trial37 and Shu et al.43. found no association between pathological response and PD-L1 expression. In our study, patients with stage III (vs I/II) or PD-L1 ≥1% (vs PD-L1<1%) were significantly more likely to achieve MPR, and histology (SCC vs non-SCC) was not significantly associated with MPR.
There were certain limitations to this meta-analysis. First of all, no prospective study data directly confirmed the correlation between pathological response and survival of neoadjuvant immunotherapy, most of the included trials were nonrandomized single-arm clinical trials with a small sample size, and these corresponding analyses were based on indirect comparisons. Second, so far, there was still no standardized method for MPR evaluation, especially when immunotherapy was added into neoadjuvant therapy. An ideal observation index should be easy to measure, and with a smaller chance of having a deviation, whereas neoadjuvant immunotherapy was different from the other treatments with more diverse pathological changes. Third, in several studies, the criteria of MPR were not quite uniform. In Checkmate-816 trial, the evaluation of MPR included sampled lymph nodes. Fourth, MPR might be differ after neoadjuvant immunotherapy between squamous cell carcinoma and adenocaicinoma, although there was no difference in our result, the optimal cutoff value for pathological response might require further refinement. Additionally, optimized methods of histologic sampling, interobserver variability in the assessment of pathological response, varied cycles of neoadjuvant therapy would affect the final pathological results. What’s more, some of the included trials are still ongoing with only initial results, more prospective studies are needed to confirm its validity and its relationship with DFS and OS. Despite the above deficiencies, to our knowledge, this is the first meta-analysis to evaluate MPR as a surrogate marker of improved long-term outcomes of NSCLC receiving immunotherapy. Once a recognized surrogate endpoint is established, it would accelerate clinical trials and drug development.
Conclusions
Results of this systematic review and meta-analysis demonstrate that nCIT achieved higher MPR in NSCLC patients, and increased MPR might be associated with survival benefits. It seems that the MPR could serve as a surrogate endpoint of survival to evaluate neoadjuvant immunotherapy. In the future, more randomized clinical trials are warranted to confirm our conclusions.
Ethical approval
No.
Conflicts of interest disclosure
The authors have no conflicts of interest.
Sources of funding
No.
Author contribution
Y.C. and J.Q.: methodology, software, validation, investigation, resources, data curation, writing-original draft; Y.W.: methodology, software, investigation, data curation; Q.L.: conceptualization, investigation, resources, data curation, writing-review and editing; J.W. and W.Z.: conceptualization, investigation, data curation; F.L.: conceptualization, methodology, software and validation; Z.H.: conceptualization, data curation, investigation; M.Z.: conceptualization, data curation, investigation; J.W.: conceptualization, methodology, validation, investigation, data curation, writing-review and editing, visualization, supervision, project administration, funding acquisition.
Guarantor
Jun Wang.
Supplementary Material
Footnotes
Y.C. and J.Q. are co-authors and contribute equally to this article and share first authorship.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 26 May 2023
Contributor Information
Yujia Chen, Email: w1048832039@163.com.
Jianjun Qin, Email: qinjianjun@cicams.ca.cn.
Yajing Wu, Email: wjzs19881212@163.com.
Qiang Lin, Email: linqiang@csco.org.cn.
Jianing Wang, Email: 20203829@stu.hebmu.edu.cn.
Wei Zhang, Email: zyida096@163.com.
Fei Liang, Email: liangfei0726@163.com.
Zhouguang Hui, Email: drhuizg@163.com.
Min Zhao, Email: z_mxk1067@126.com.
Jun Wang, Email: wangjun0818@hebmu.edu.cn.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 3. al-Kattan K, Sepsas E, Fountain SW, et al. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 1997;12:380–384. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet 2000;355:479–485. [DOI] [PubMed] [Google Scholar]
- 5. Carnio S, Novello S, Papotti M, et al. Prognostic and predictive biomarkers in early stage non-small cell lung cancer: tumor based approaches including gene signatures. Transl Lung Cancer Res 2013;2:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentrerandomised trial and update of systematic review. Lancet 2007;369:1929–1937. [DOI] [PubMed] [Google Scholar]
- 7. Gutierrez-Sainz L, Cruz-Castellanos P, Higuera O, et al. Neoadjuvant chemoimmunotherapy in patients with resectable non-small cell lung cancer. Curr Treat Options Oncol 2021;22:91. [DOI] [PubMed] [Google Scholar]
- 8. Liang W, Cai K, Chen C, et al. Expert consensus on neoadjuvant immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2020;9:2696–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weissferdt A, Pataer A, Vaporciyan AA, et al. Agreement on major pathological response in nsclc patients receiving neoadjuvant chemotherapy. Clin Lung Cancer 2020;21:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hellmann MD, Chaft JE, William WN, Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 13. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cascone T, William WN, Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Provencio M, Serna-Blasco R, Nadal E, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage iiia non–small-cell lung cancer (NADIM phase II trial). WCLC 2022;40:2924–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng Y, Sun W, Zhang J, et al. Neoadjuvant PD-1 inhibitor combines with chemotherapy versus neoadjuvant chemotherapy in resectable squamous cell carcinoma of the lung. Thorac Cancer 2022;13:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lei J, Yan X, Zhao J, et al. A randomised, controlled, multicenter phase II trial of camrelizumab combined with albumin-bound paclitaxel and cisplatin as neoadjuvant treatment in locally advanced NSCLC. Ann Oncol 2020;31:S1441–S1442. [Google Scholar]
- 18. Altorki NK, McGraw TE, Borczuk AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol 2021;22:824–835. [DOI] [PubMed] [Google Scholar]
- 19. Qiu FM. Two cycles versus three cycles of neoadjuvant sintilimab plus platinumdoublet chemotherapy in patients with resectable non-small-cell lung cancer (neoSCORE): a randomized, single center, two-arm phase II trial. ASCO:oral 2022;14:8500. [Google Scholar]
- 20. Hou X, Shi X, Luo J. Efficacy and safety of camrelizumab (a PD-1 inhibitor) combined with chemotherapy as a neoadjuvant regimen in patients with locally advanced non-small cell lung cancer. Oncol Lett. 2022;24:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang H, Yang C, Gonzalez-Rivas D, et al. Sleeve lobectomy after neoadjuvant chemoimmunotherapy/chemotherapy for local advanced non-small cell lung cancer. Transl Lung Cancer Res 2021;10:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Z, Gao Z, Zhang M, et al. Real-world effectiveness and prognostic factors analysis of stages i-iii non-small cell lung cancer following neoadjuvant chemo-immunotherapy or neoadjuvant chemotherapy. Ann Thorac Cardiovasc Surg 2022;28:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao D, Xu L, Wu J, et al. Comparison of perioperative outcomes among non-small cell lung cancer patients with neoadjuvant immune checkpoint inhibitor plus chemotherapy, EGFR-TKI, and chemotherapy alone: a realworld evidence study. Transl Lung Cancer Res 2022;11:1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Z, Wu Z, Qin Y, et al. Perioperative safety and feasibility outcomes of stage IIIA-N2 non-small cell lung cancer following neoadjuvant immunotherapy or neoadjuvant chemotherapy: a retrospective study. Ann Transl Med 2021;9:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaft JE, Oezkan F, Kris MG, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med 2022;28:2155–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Liu S, Yang L, et al. Camrelizumab combined with albumin paclitaxel and platinum in perioperative treatment of resectable squamous cell lung cancer: a single-arm, open-label, phase II clinical trial. Ann Oncol 2022;33:S978. [Google Scholar]
- 27. Bahce I, Dickhoff C, Schneiders FL, et al. Ipilimumab plus nivolumab and chemoradiotherapy followed by surgery in patients with resectable and borderline resectable lung cancer: the increase trial. Ann Oncol 2022;33:S982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao Y, Jiang J, Xiao D, et al. Robotic-assisted thoracic surgery following neoadjuvant chemoimmunotherapy in patients with stage iii non-small cell lung cancer. Front Oncol. 2022;12:969545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin YB, Long H, Chen YH, et al. Phase II trial of neoadjuvant tislelizumab with chemotherapy for resectable stage iib-iii non-small cell lung cancer. J Thorac Oncol. 2022;17:S287. [Google Scholar]
- 30. Yan S, Chen J, Wang J, et al. Neoadjuvant toripalimab combination in patients with stage IIBIIIB NSCLC: a single-arm, phase 2 trial (renaissance study). J Thorac Oncol. 2022;17:S288–9. [Google Scholar]
- 31. Zhang Y, Zeng L, Zhang X, et al. An updated analysis of toripalimab and platinum-doublet chemotherapy as neoadjuvant therapy for potentially resectable NSCLC. J Thorac Oncol. 2022;17:9(S291). [Google Scholar]
- 32. Wang J, Li J, Cai L, et al. The safety and efficacy of neoadjuvant programmed death 1 inhibitor therapy with surgical resection in stage IIIA non-small cell lung cancer. Ann Transl Med 2021;9:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duan H, Wang T, Luo Z, et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: an open-label, multicenter, single-arm study. Transl Lung Cancer Res 2021;10:1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Zeng L, Zhang X, et al. Efficacy and biomarker identification of neoadjuvant chemo-immunotherapy in potentially resectable non-small cell lung cancer. Ann Oncol 2021;32:S934. [Google Scholar]
- 35. Zinner R, Axelrod R, Solomides CC, et al. Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin /gemcitabine (G) in resectable NSCLC. J Clin Oncol 2020;38:suppl9051. [Google Scholar]
- 36. Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413–1422. [DOI] [PubMed] [Google Scholar]
- 37. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen D, Wang J, Wu J, et al. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J Thorac Dis 2021;13:1760–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eichhorn F, Klotz LV, Kriegsmann M, et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: First clinical experience. Lung Cancer 2021;153:150–157. [DOI] [PubMed] [Google Scholar]
- 41. Bar J, Urban D, Redinsky I, et al. Neoadjuvant pembrolizumab for early stage non-small cell lung cancer. J Thorac Oncol 2021;16:S865–S866. [Google Scholar]
- 42. Tong BC, Gu L, Wang X, et al. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2022;163:427–436. [DOI] [PubMed] [Google Scholar]
- 43. Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786–795. [DOI] [PubMed] [Google Scholar]
- 44. Lee J, Chaft J, Nicholas A, et al. Surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB-IIIB NSCLC: LCMC3 trial primary analysis. J Thorac Oncol 2021;16:S59–S61. [Google Scholar]
- 45. Zhang P, Dai J, Sun F, et al. Neoadjuvant sintilimab and chemotherapy for resectable stage IIIA non-small cell lung cancer. Ann Thorac Surg 2022; Sep;114:949–958. [DOI] [PubMed] [Google Scholar]
- 46. Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816–826. [DOI] [PubMed] [Google Scholar]
- 47. Tao X, Li N, Wu N, et al. The efficiency of 18F-FDG PET-CT for predicting the major pathologic response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2020;47:1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu X, Sun L, Song N, et al. Neoadjuvant PD-1 inhibitor (toripalimab) plus chemotherapy in patients with potentially resectable NSCLC: an open-label, single-arm, phase II trial. Ann Oncol 2021;32:S942. [Google Scholar]
- 49. Wu YL, Chen KN, Xing WQ, et al. SHR-1316 vs placebo in combination with chemotherapy as perioperative treatment in patients with resectable stage II–III NSCLC: a randomized, double-blind, multicenter, phase 1b/3 trial. Ann. Oncol 2022;33:S72. [DOI] [PubMed] [Google Scholar]
- 50. Zhao ZR, Yang CP, Chen S, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology 2021;10:1996000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hong MH, Ahn B, Kim HR, et al. Interim analysis of neoadjuvant chemoradiotherapy and durvalumab for potentially resectable stage III non-small cell lung cancer (NSCLC). J Thorac Oncol 2021;16:S194–S195. [Google Scholar]
- 52. Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-a multicenter single-arm phase II trial. J Clin Oncol 2021;39:2872–2880. [DOI] [PubMed] [Google Scholar]
- 53. Tfayli A, Al Assaad M, Fakhri G, et al. Neoadjuvant chemotherapy and Avelumab in early stage resectablenonsmall cell lung cancer. Cancer Med 2020;9:8406–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deng H, Liu J, Cai X, et al. Radical minimally invasive surgery after immuno-chemotherapy in initially-unresectable stage IIIB non-small cell lung cancer. Ann Surg 2022;275:e600–e602. [DOI] [PubMed] [Google Scholar]
- 55. Shi L, Liu Z, Meng Q, et al. Pathologic response to neoadjuvant PD-1 inhibitors and chemotherapy in squamous non-small-cell lung cancer. J Thorac Oncol 2021;16:S979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hong T, Sun T, Zhang M, et al. Surgical perspective in neoadjuvant chemoimmunotherapy for stage II–III non-small cell lung cancer. Thorac Cancer 2021;12:2796–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hu Y, Ren SY, Wang RY, et al. Surgical outcomes after neoadjuvant chemoimmunotherapy for resectable non-small cell lung cancer. Front Oncol 2021;11:684070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng X, Huang J, Zhou M, et al. EP02.04-010 clinical outcomes after neoadjuvant tislelizumab plus chemotherapy in resectable stage IIIA-B NSCLC: a retrospective study. J Thor Oncol 2022;17:S236–S237. [Google Scholar]
- 59. Chen T, Ning J, Campisi A, et al. Neoadjuvant PD-1 inhibitors and chemotherapy for locally advanced nsclc: a retrospective study. Ann Thorac Surg 2022;113:993–999. [DOI] [PubMed] [Google Scholar]
- 60. Wu J, Hou L, Haoran E, et al. Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer 2022;165:115–123. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Y, Zeng L, Zhang X, et al. The efficacy and safety of neoadjuvant anti-PD-1 inhibitor combined with platinum-based chemotherapy in patients with potentially resectable non-small cell lung cancer. J Clin Oncol 2021;39:e20526. [Google Scholar]
- 62. Chen Y, Yan B, Xu F, et al. Neoadjuvant chemoimmunotherapy in resectable stage IIIA/IIIB non-small cell lung cancer. Transl Lung Cancer Res 2021;10:2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhai H, Li W, Jiang K, et al. Neoadjuvant nivolumab and chemotherapy in patients with locally advanced non-small cell lung cancer: a retrospective study. Cancer Manag Res 2022;14:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yao Y, Tang D, Gao W, et al. Neoadjuvant immuno-chemotherapy: a new perspective for stage III NSCLC? Front Surg 2022;9:843987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fan BS, Wang XT, Di SY, et al. Short-term outcomes of neoadjuvant sintilimab with chemotherapy in stage III non-small cell lung cancer: a case series. Transl Cancer Res 2022;11:1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stefani D, Plönes T, Viehof J, et al. Lung cancer surgery after neoadjuvant immunotherapy. Cancers (Basel) 2021;13:4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Broderick SR, Bott MJ. Neoadjuvant immunotherapy in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1471–1474. [DOI] [PubMed] [Google Scholar]
- 68. Brandt WS, Yan W, Zhou J, et al. Outcomes after neoadjuvant or adjuvant chemotherapy for cT2-4N0-1 non-small cell lung cancer: a propensity-matched analysis. J ThoracCardiovasc Surg 2019;157:743–753 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ulas EB, Dickhoff C, Schneiders FL, et al. Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: a systematic review. ESMO Open 2021;6:100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer 2020;2:zcaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 72. Mouillet G, Monnet E, Milleron B, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol 2012;7:841–849. [DOI] [PubMed] [Google Scholar]
- 73. Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med 2021;27:301–309. [DOI] [PubMed] [Google Scholar]
- 74. Waser NA, Adam A, Schweikert B, et al. 1243P Pathologic response as early endpoint for survival following neoadjuvant therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC): Systematic literature review and meta-analysis. Ann Oncol 2020;31(Suppl_4). [Google Scholar]
- 75. William WN, Jr, Pataer A, Kalhor N, et al. Anderson Lung Cancer Collaborative Research Group. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015;33:3541–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yi JS, Ready N, Healy P, et al. Immune activation in early-stage non-small cell lung cancer patients receiving neoadjuvant chemotherapy plus ipilimumab. Clin Cancer Res 2017;23:7474–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009;58:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol 2019;30:385–396. [DOI] [PubMed] [Google Scholar]
- 80. Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535–554. [DOI] [PubMed] [Google Scholar]
- 81. Zhang B, Xiao H, Pu X, et al. A real-world comparison between neoadjuvant chemoimmunotherapy and chemotherapy alone for resectable non-small cell lung cancer. Cancer Med 2022;12:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–3559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


