Fig. 1.
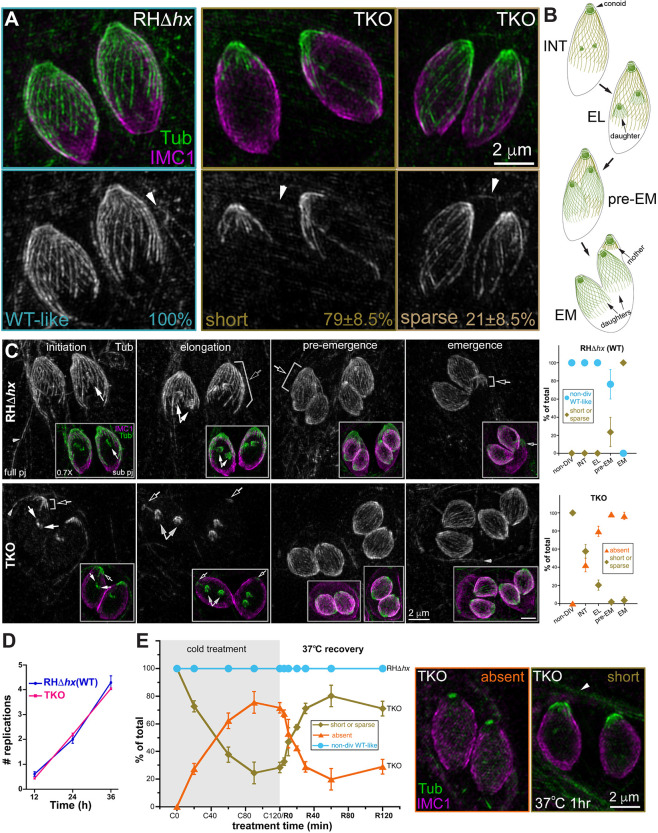
Cortical microtubules in intracellular mature TKO and wild-type parasites. (A) Projections of image stacks acquired by three-dimensional structured illumination microscopy (3D-SIM) of the non-dividing wild-type (WT; RHΔhx) and TKO (Δtlap2Δspm1Δtlap3) parasites. Whereas 100% of the wild-type parasites have the typical spirally arranged full microtubule array, ∼79±8.5% (mean±s.d.; three independent experiments) of the TKO parasites have significantly curtailed/short microtubules. The cortical microtubules are sparse in the other 21%. Green and grayscale, tubulin (Tub) labeling by a mouse anti-acetylated tubulin antibody, which also labels the microtubules in the host cell (arrowhead). Magenta, cortex labeling by a rabbit anti-IMC1 antibody. Arrowheads, host cell microtubules. (B) Diagrams of wild-type parasites at the initiation (INT), elongation (EL), pre-emergence (pre-EM) and emergence (EM) stages of daughter construction. Cortical microtubules and the conoid are shown for the mother and daughter parasites. (C) Left, projections of 3D-SIM images of TKO and wild-type parasites at the initiation, elongation, pre-emergence, and emergence stage of daughter construction. Black arrows, cortical microtubules in the mother parasite. White arrows, daughter parasites. Arrowheads, host-cell microtubules. Insets (0.7×) are projections that include a subset of the image stack (sub pj) to better display the structure of the daughter parasites, which are obscured in the full projections (full pj) for the wild-type parasites due to the cortical microtubules in the mother. Note that for the TKO parasite, due to the shortness or complete absence of the maternal microtubules, the daughters are as fully visible in the full projection as in the subset projection. Right, quantification of the proportions of wild-type (top) and TKO (bottom) parasites that display various characteristics in the maternal cortical microtubule array at different stages, including the non-dividing (non-DIV) stage or the initiation (INT), elongation (EL), pre-emergence (pre-EM), and emergence (EM) stages of daughter construction. For the TKO parasites, the two patterns observed are ‘short or sparse’ (i.e. similar to non-dividing TKO parasites) and ‘absent’. For the wild-type parasites, the two patterns observed are ‘non-dividing wild-type-like’ or ‘short or sparse’. Error bars are s.e.m. from three independent experiments. Total number of parasites analyzed were: 88, 68, 50 and 36 for TKO, and 52, 62, 54 and 49 for wild type at the initiation, elongation, pre-emergence and emergence stage, respectively. The data for the non-dividing stage is the same as shown in Fig. 1A. (D) Average number of cell divisions at 12, 24 or 36 h after infection in four independent experiments for RHΔhx (WT) and TKO parasites. Error bars show the s.d. (E) Left, quantification of the percentage of non-dividing wild-type (RHΔhx) and TKO parasites that display various characteristics in the array of the cortical microtubules after being placed at ∼7°C for 0, 20, 60, 90 or 120 min (C0–C120) as well as after recovery at 37°C for 5, 10, 20, 30, 60 or 120 min after 120 min of∼7°C incubation (R5–R120). For the TKO parasites, the two patterns observed are ‘short or sparse’ or ‘absent’. For wild-type parasites, the cortical microtubules remain similar to those in untreated parasites under all conditions tested here (after cold treatment and cold treatment plus 37°C recovery). Error bars are s.e.m. from three independent experiments. Middle, projection of 3D-SIM images of TKO parasites in which cortical microtubules are absent. Right, projection of 3D-SIM images of TKO parasites after 1 h of 37°C (R60) recovery, in which short cortical microtubules at the apical end are seen. Arrowhead, host-cell microtubules.