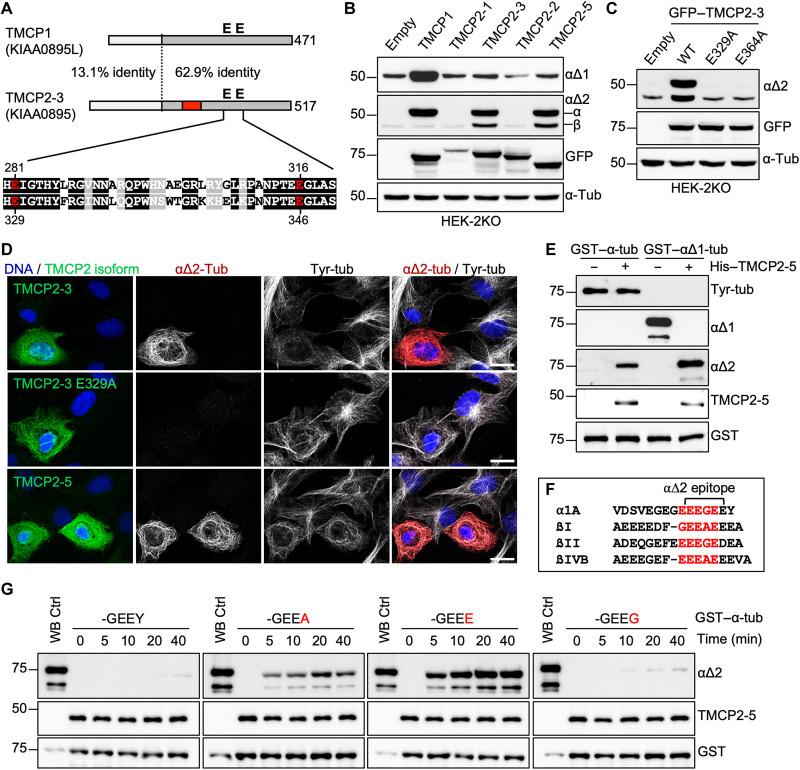
Fig. 4. Characterization of TMCP2, a paralog of TMCP1.
(A) Schematic alignment of TMCP1 and TMCP2 (isoform 3) shows a high degree of conservation in the C-terminal region that contains the active site. The region encoded by the alternative exon is indicated in red. Sequence alignment of the active site region with highlighted essential glutamates is presented below. (B) Immunoblots of protein extracts from HEK-2KO cells expressing TMCP1 and several isoforms of TMCP2. (C) Immunoblots of protein extracts from HEK-2KO cells expressing TMCP2-3 or its enzymatically inactive versions (E329A and E364A). (D) Immunofluorescence analysis of RPE1 cells expressing TMCP2-3 or its enzymatically inactive version (E329A) and TMCP2-5. Scale bars, 20 μm. (E) Immunoblots of an in vitro assay involving recombinant His-TMCP2-5 and bacterially produced GST–α-tubulin or GST–αΔ1-tubulin. (F) Schematic representation of the potential epitopes (highlighted in red) recognized by αΔ2 antibody present in the C terminus of α-, βI-, βII-, and βIV-tubulin. (G) Immunoblots of an in vitro time-course assay using recombinant His-TMCP2-5 in the presence of either the WT GST–α-tubulin or its mutated versions in which the C-terminal tyrosine has been replaced by alanine, glutamate, or glycine.