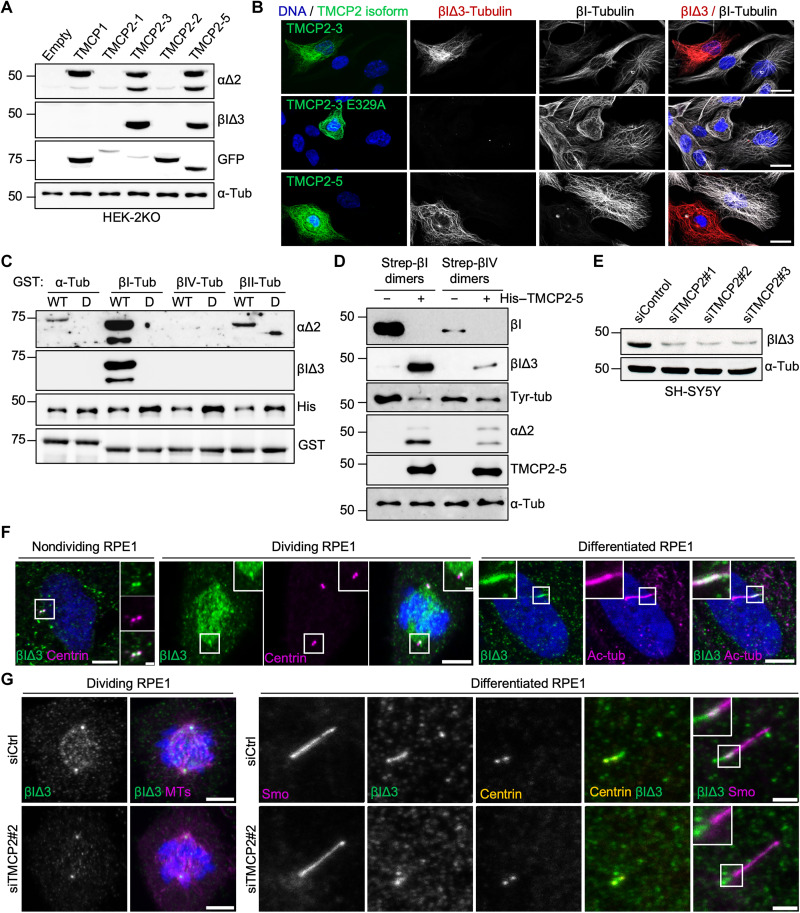
Fig. 5. TMCP2 catalyzes previously unknown βIΔ3 modification.
(A) Immunoblots of protein extracts from HEK-2KO cells expressing TMCP1 and several isoforms of TMCP2 probed with the indicated antibodies, including the newly generated anti–βIΔ3-tubulin antibody. (B) Immunofluorescence analysis of RPE1 cells expressing TMCP2-3 or its enzymatically inactive version (E329A) and TMCP2-5 using anti–βI-tubulin or anti–βIΔ3 antibody. Scale bars, 20 μm. (C) Immunoblots of an in vitro assay involving recombinant TMCP2-5 or its enzymatically inactive version and bacterially produced GST-α-, βI-, βII-, and βIV-tubulin. (D) Immunoblot analysis of an in vitro assay involving recombinant TMCP2-5 and tubulin dimers enriched for βI- or βIV-tubulin after Strep-tag–based purification from HEK293 cells. (E) Immunoblots of protein extracts from SH-SY5Y cells following knockdown of TMCP2 using three different siRNAs. (F) Immunofluorescence analysis of endogenous βIΔ3-tubulin in nondividing, mitotic, or differentiated RPE1 cells. βIΔ3-tubulin is enriched at centrioles and mitotic spindles as well as primary cilia. Scale bars, 5 μm; inset, 1 μm. (G) Immunofluorescence analysis of either dividing or differentiated RPE1 cells knockdown for TMCP2. Scale bars, 5 μm for dividing RPE1 and 2 μm for differentiated RPE1.