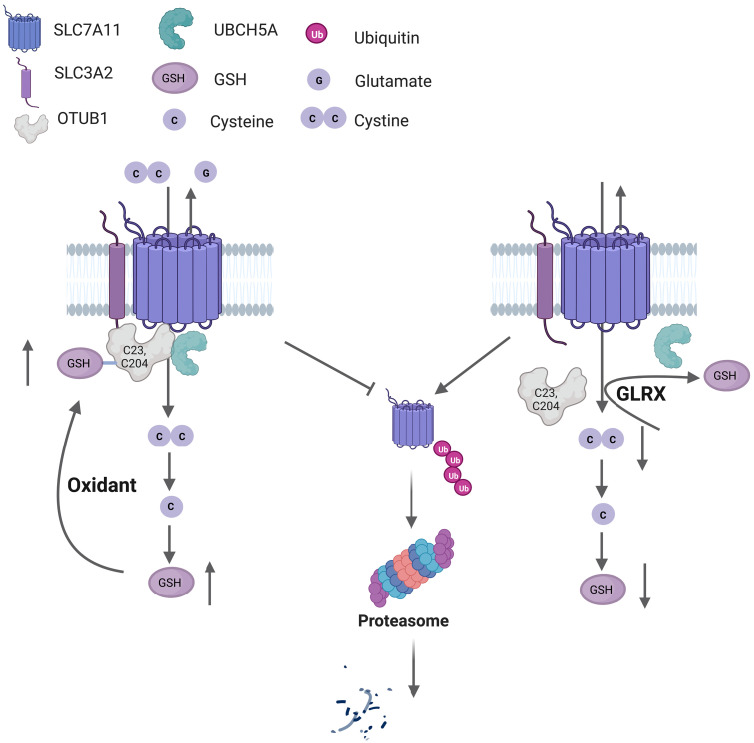
Fig. 10. Graphical abstract depicting system xC− regulation in a redox-dependent manner through OTUB1-SSG.
S-glutathionylation of the deubiquitinase OTUB1 promotes stabilization of SLC7A11, the active subunit of system xC− transporter. An enhanced oxidative environment leads to increases in S-glutathionylation of OTUB1 (OTUB1-SSG), at C23 and C204, which increases interaction with the E2 Ub-conjugating enzyme, UBCH5A, to prevent ubiquitination and proteasomal degradation of SLC7A11. The resultant increase in system xC− transporter activity allows enhanced cystine uptake that will be incorporated in GSH synthesis following its reduction to cysteine intracellularly (left). The deglutathionylase, GLRX, removes GSH from OTUB1, leading to ubiquitination of SLC7A11 and its subsequent proteasomal degradation, resulting in lower GSH content (right). This pathway represents a feed-forward regulatory mechanism whereby enhanced GSH-dependent protein oxidation augments cellular GSH. The figure was created using BioRender.com.