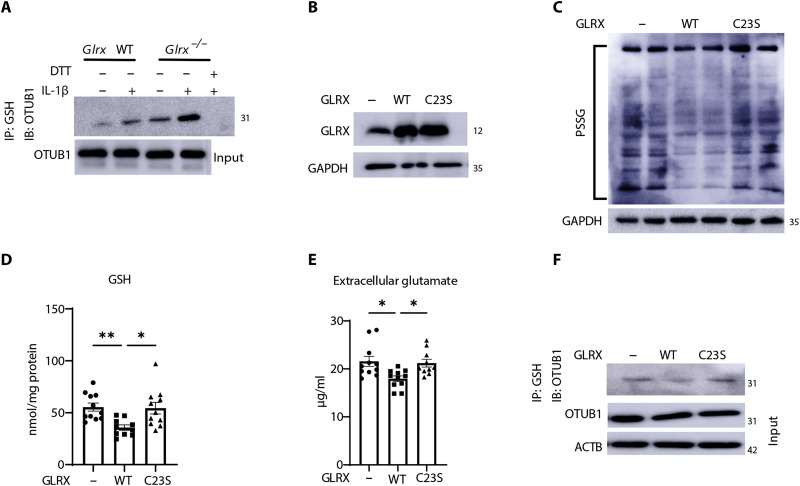
Fig. 4. OTUB1 is a target for S-glutathionylation and is regulated by GLRX.
(A) Detection of OTUB1-SSG in WT or Glrx−/− MTE cells stimulated with vehicle or IL-1β for 24 hours. PSSG was immunoprecipitated with anti-GSH followed by Western blotting of OTUB1. DTT was incubated with cell lysates before immunoprecipitation as a negative control. OTUB1 levels from whole-cell lysates were used as the input control. (B) Evaluation of GLRX in lysates prepared from cells 5 days after treatment with WT-GLRX or C23S-mutant GLRX recombinant protein. GAPDH, loading control. (C) Assessment of total PSSG in WT MTE cells treated with WT GLRX or C23S-GLRX recombinant protein. Shown are nonreducing Western blots probed with anti-GSH antibody. GAPDH, loading control. (D) GSH levels in MTE cells treated with recombinant WT-GLRX or C23S-GLRX. (E) Glutamate in cell culture supernatant of MTE cells treated with WT-GLRX or C23S-GLRX. (F) Detection of OTUB1-SSG in cells treated with recombinant WT-GLRX or C23S-GLRX protein following immunoprecipitation of PSSG using GSH antibody, followed by Western blotting analysis of OTUB1. Total OTUB1 levels from whole-cell lysates were used as the input control. *P < 0.05 and **P < 0.01. IB, immunoblot.