Abstract
Abemaciclib is an orally administered, potent, and selective small molecule inhibitor of cyclin‐dependent kinases 4 and 6, approved for advanced or metastatic breast cancer. This study aimed to use an exposure–response approach to investigate the effect of abemaciclib and its active metabolites (M2 and M20) on QTc interval and delay in cardiac repolarization at clinically relevant exposures. This was a single‐blind, randomized, and placebo‐controlled study of ascending doses of abemaciclib. Thirty‐five healthy participants were administered a single dose of 200–600 mg abemaciclib. Twelve‐lead electrocardiogram tracings and pharmacokinetic samples were collected serially pre‐ and post‐dose. The primary objective was to study the relationship between abemaciclib and its active metabolites (M2 and M20) and QTc interval following ascending oral doses of abemaciclib. The secondary objective included evaluating the safety and tolerability of single ascending doses of abemaciclib in healthy participants. Exposure–response analysis demonstrated that there was no significant relationship between placebo‐corrected change from baseline QTcF (ΔΔQTcF), abemaciclib, and metabolite plasma concentrations. Additionally, the ΔΔQTcF slopes of abemaciclib, its metabolites, and total analyte concentrations were not statistically different from zero. Single doses of abemaciclib, up to 400 mg, were well‐tolerated by healthy participants; however, at the 600 mg dose (three times the highest registered dose), the frequency and severity of treatment‐related gastrointestinal events (primarily diarrhea, nausea, and vomiting) increased. In conclusion, single doses of abemaciclib, up to 400 mg, had no statistically or clinically relevant effects on QTc, and abemaciclib was well tolerated up to a dose of 400 mg in this study.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Currently, information on abemaciclib's effect on QTc interval is limited, though many anticancer drugs are associated with QT prolongation.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study aimed to understand the correlation between the plasma concentration (exposure) of abemaciclib and its metabolites and the subsequent effect (response) on cardiac repolarization (QTc interval) in healthy participants.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We demonstrate that single doses of abemaciclib up to 400 mg had no statistically or clinically relevant effects on QTc, and abemaciclib was well tolerated up to a dose of 400 mg.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study highlights the benefit of addressing potential QTc prolongation risk by exposure–response analysis, which is a suitable approach if traditional thorough QT studies are not feasible. At the time of the study, abemaciclib's effects were only known in cancer patients. However, our study concluded that the drug was safe to use in healthy participants.
INTRODUCTION
Abemaciclib is an orally administered, potent, and selective small molecule inhibitor of cyclin‐dependent kinases (CDKs) 4 and 6, approved for advanced or metastatic breast cancer. Inhibition of CDK4 and CDK6 prevents cell cycle progression through the G1 restriction point that controls entry into S phase, thus arresting tumor growth. 1 The International Conference on Harmonisation (ICH) has recommended that drugs in clinical development be subject to rigorous evaluation of their potential to prolong the QT interval. Until recently, a thorough QT/corrected QT (QTc) study (TQT study) design with therapeutic and/or supratherapeutic doses was standardized per the E14 guidance.
Upon comparisons of data from TQT studies and the IQ‐CRSC study, 2 , 3 a revision of the E14 guidance (R3) described that an intensive electrocardiogram (ECG) and pharmacokinetic (PK) sampling schedule in an early‐phase clinical trial evaluating a range of doses would provide data with a similar level of confidence as a ‘traditional’ TQT study. 3 , 4 , 5 , 6 This exposure–response (ER) approach within an early‐phase study offers significant benefits over the traditional TQT study design in that ER analysis improves the precision of the QT effect as the model delineates the effect for all data across a range of plasma concentrations. 2
In oncology, toxicity concerns typically prevent the use of a traditional TQT study design with therapeutic and supratherapeutic doses of the investigational compound. For abemaciclib, a single ascending dose (SAD) study in healthy participants was selected to determine tolerability, prior to administering a “drug–drug interaction (DDI)‐determined” dose. Due to the evolving regulatory paradigm (including the E14[R3] revision), the use of the ER approach within the SAD study allowed for the use of a smaller sample size, and therefore fewer healthy participants exposed to higher doses of abemaciclib.
A primary ER analysis based on paired ECG and PK samples was planned to evaluate the relationship between the concentration of abemaciclib and its active metabolites (with exposures >10% of total drug‐related exposures) and QTc interval and delayed cardiac repolarization.
In previous studies, abemaciclib had been shown to slightly change heart rate (HR), 7 by up to 4.3 beats per minute (bpm), in a pilot food effect study when abemaciclib was administered in the fasting state. In a prior DDI study, when abemaciclib was combined with rifampin, HR increased approximately 11 bpm 6 h post‐dose, and 9 bpm 10 h post‐dose. However, no HR changes occurred when abemaciclib was administered alone. Although these changes in HR were not statistically significant, in this study the selection of the correction method was planned based on the observed change in HR. Fridericia correction was prospectively planned to be used if the ΔΔHR did not exceed 10 bpm at any time post‐dose; whereas beat‐to‐beat analysis was to be used if ΔΔHR exceeded 10 bpm.
METHODS
Clinical study design
A randomized, single‐blind, placebo‐controlled, single‐ascending‐dose crossover study was conducted at two sites in the United States. Thirty‐five participants were enrolled into two cohorts: Cohort 1, comprising 20 participants, was conducted at Covance Clinical Research Unit (CRU) Daytona Beach (FL, USA) and Cohort 2 comprising 15 participants was conducted at Covance CRU Evansville (IN, USA). Illustrations of the study design are shown in Figure 1 and a Consolidated Standards of Reporting Trials (CONSORT) flowchart is shown in Figure S1. An independent ethical review board approved the trial protocol and any subsequent amendments. Study participants provided informed consent prior to study enrollment. The trial was registered at ClinicalTrials.gov (NCT02677844). The first patient was enrolled on February 10, 2016, and the last patient completed follow‐up on July 12, 2016.
FIGURE 1.
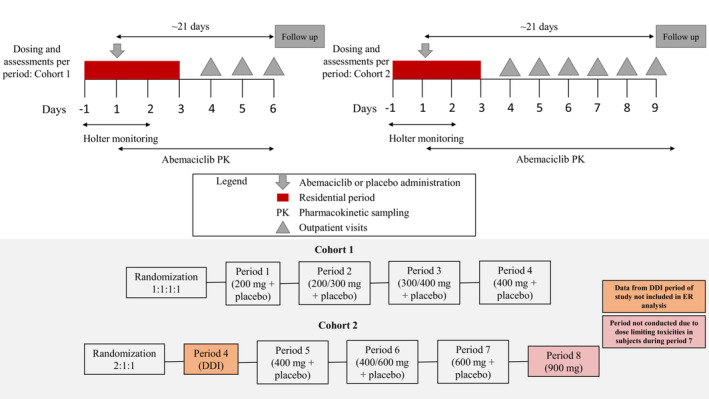
Illustration of the study design. DDI, drug–drug interaction; ER, exposure–response.
The study was planned to evaluate the relationship between QT interval and exposure after oral administration of single ascending doses of abemaciclib 200–400 mg (Cohort 1) and 600–900 mg (Cohort 2). Inclusion criteria accepted healthy, sterile males and females of non‐childbearing potential, aged 18–70 years, with a body mass index (BMI) between 18 and 32 kg/m2 with acceptable clinical laboratory test results and acceptable blood pressure (Table S1 outlines baseline blood pressure, pulse rate, and creatinine serum levels) as determined by the investigator were eligible for this study. Participants excluded from the study included those who had an abnormality in the 12‐lead ECG that, in the opinion of the investigator, increased the risks associated with participating in the study or affected or confounded the corrected QT interval (QTc analysis).
Participants in Cohort 1 were randomized to one of four sequences in a 1:1:1:1 ratio and received study drug as a single oral dose on Day 1 of each of four separate, consecutive periods, according to the randomization. A washout of at least 5 days was required between doses, during which time a review of data was conducted for dose escalation decisions. Participants were admitted to the CRU on Day −1 of each period. On Day 1, approximately 2 h before study drug administration, participants began continuous 12‐lead digital Holter monitoring which continued until 24 h post‐dose. Blood samples were collected for the measurement of plasma concentrations of abemaciclib, M2, and M20 during the 24‐h Holter monitoring period (Days 1 and 2). Participants were discharged from the CRU following safety assessments and PK sampling on Day 3 (48 h after dosing) and returned to the CRU for safety assessments and/or PK sampling on Days 4, 5, and 6 of the period.
A secondary objective of the study was to evaluate the effect of abemaciclib on the PK of a P‐gp substrate because in vitro data indicated abemaciclib may inhibit P‐gp. Loperamide was selected as the probe P‐gp substrate because it is commonly used in patients taking abemaciclib. Period 4 of Cohort 2 was designed to evaluate the DDI between a 400 mg dose of abemaciclib and the P‐gp substrate loperamide. The results of this part of the study have been reported previously. 8 Due to safety‐related events occurring during the DDI period, the Safety Review Panel decided that the 400 mg dose level should be repeated; thus, the randomization was adjusted to reflect this dose modification throughout the remaining periods.
Participants in Cohort 2 were randomized to one of three sequences (2:1:1 ratio) to receive study drug as a single oral dose on Day 1 of separate, consecutive periods (Periods 5–7). While admitted in the CRU, participants in Cohort 2 underwent the same procedures as described for participants in Cohort 1 and were discharged following safety assessments and PK sampling on Day 3 (48 h after dosing). Participants in Cohort 2 returned to the CRU for safety assessments and/or PK sampling on Days 4, 5, 6, 7, 8, and 9.
Pharmacodynamic analyses
Continuous ECG monitoring was conducted for each participant using a 12‐lead digital Holter recorder from approximately 2 h pre‐dose through 24 h post‐dose on Day 1 of each period. Participants were required to rest for 8 h post‐dose in a semi‐supine position, minimizing exertion and external stimuli. After the 8‐h post‐dose period, participants were allowed to ambulate. At all times during the Holter monitoring period, participants were required to lie supine in a quiet environment for 10 min before and 5 min after each ECG measurement. Participants underwent an overnight fast of at least 10 h before drug administration with approximately 240 mL of water. Food was restricted until at least 4 h before dosing, after which participants were given a meal. Water was restricted 1 h before and 1 h after dosing. Otherwise, participants were allowed water as desired, and meals were provided at appropriate times. PK samples were taken after ECG recording.
The continuous Holter recordings were transferred to a designated central ECG laboratory (Biomedical Systems Corporation) where a cardiologist (blinded to participant, visit, and treatment allocation) conducted a full over‐read (including the measurements of all intervals) upon all extracted ECGs for each participant. The central ECG laboratory over‐read was used for data analysis. ECG data from participants administered loperamide in Period 4 of Cohort 2 were not included in the analysis to avoid confounding the results.
ECG extraction and analysis
Up to 10 14‐s digital 12‐lead ECG tracings were extracted from the continuous Holter recordings at −2, −1.5, −1, −0.5, and −0.25 h prior to dosing, and at 2, 4, 6, 8, 10, 12, 14, and 24 h after dosing using the ‘TQT Plus method,’ a computer‐assisted and statistical process (iCardiac Technologies). At each protocol‐specified timepoint, 10 ECG replicates were extracted from a 5‐min ‘ECG window’ (typically, the last 5 min of the 15‐min period when the participant was maintained in a supine or semi‐recumbent quiet position).
The central ECG laboratory performed quality control checks and initial assessment of QT corrections using data recorded during the 2‐h pre‐dose monitoring on Day 1. The central ECG laboratory reviewed the 2‐h pre‐dose Holter monitor recording and selected eight timepoints (in addition to five predefined timepoints) that represented good‐quality ECG segments, one when the HR was at its nadir and one at its zenith. The remainder represented a relatively even distribution of HRs from the remainder of the recording.
Pharmacokinetic analyses
Venous blood samples were collected to determine the plasma concentrations of abemaciclib, M2, and M20 at the following times relative to dosing on Day 1 of each period: pre‐dose, and 2, 4, 6, 8, 10, 12, 14, 24, 48, 72, 96, and 120 h post‐dose. Participants in Cohort 2 continued to undergo blood sample collections at 144, 168, and 192 h post‐dose.
Plasma samples obtained during this study were analyzed for abemaciclib, M2, and M20 using a validated liquid chromatography with tandem mass spectrometry (LC–MS/MS) method at Q2 Solutions located in Ithaca, NY, USA. The lower limit of quantification was 1 ng/mL, and the upper limit of quantification was 500 ng/mL for all analytes.
Noncompartmental PK analyses were conducted using Phoenix WinNonlin Version 6.4 (Certara). Abemaciclib dose proportionality was assessed by fitting the power model to area under the concentration versus time curve (AUC) (AUC [0–t last], AUC [0–∞]) and C max versus dose. 9 The 95% confidence intervals (95% CIs) of the exponent were used to assess dose proportionality.
Statistical analysis
QT values corrected for HR (QTc) were obtained using the methods described below.
Fridericia‐corrected QT interval (QTcF): , where QT and RR intervals were obtained from each replicate of ECG measurement.
QTbtb: Dynamic beat‐to‐beat QT analysis (QTbtb) employed no correction factors and took into account normal hysteresis associated with the QT interval.
Baseline and placebo effects: The average QTc of replicate QTc measurements at each timepoint following dosing were calculated for each participant and for each period. The average of the QTc across all pre‐dose timepoints (including all replicates) on Day 1 was calculated for each participant for each study period and served as the period‐specific baseline. The difference from baseline (ΔQTc) for each timepoint was determined by subtracting that participant's baseline QTc. The primary analysis was based on the time‐matched, placebo‐corrected, and baseline‐adjusted QTc (ΔΔQTc).
If a significant HR change was determined as defined by the largest mean ΔΔHR exceeding 10 bpm at any timepoint post‐dosing, it was planned that ΔΔQTbtb was to be used as the primary response variable, otherwise ΔΔQTcF was used. Categorical analyses of QTc interval data were conducted to provide number and percentage of participants meeting or exceeding defined thresholds for ECG parameters as described by ICH E14 Guidance criteria (ICH, 2005).
The primary end point of the relationship between plasma concentrations of abemaciclib, M2, and M20 and ΔΔQTcF or ΔΔQTbtb was evaluated using a linear mixed‐effects modeling approach. The response variable was either ΔΔQTcF or ΔΔQTbtb, and concentrations were fitted as a fixed effect with participant as a random effect. The intercept and slope from the model were reported together with their respective 90% CIs. Predicted mean placebo‐adjusted change from baseline ΔΔQTcF at C max was estimated with its 90% CI.
RESULTS
Demographics and disposition
Overall, a total of 35 healthy participants, 7 males and 28 females, between the ages of 32 and 70 years, participated in this study. Detailed demographics are shown in Table 1.
TABLE 1.
Participant demographics and baseline characteristics.
| Parameter | Cohort 1 (N = 20) | Cohort 2 (N = 15) | Overall (N = 35) | |
|---|---|---|---|---|
| Age (years) | Mean (SD) | 48.9 (7.9) | 58.0 (7.5) | 52.8 (8.9) |
| Range | 32–59 | 43–70 | 32–70 | |
| Sex (N) | Male | 5 | 2 | 7 |
| Female | 15 | 13 | 28 | |
| Ethnicity (N) | Hispanic or Latino | 10 | 0 | 10 |
| Not Hispanic or Latino | 10 | 15 | 25 | |
| Race (N) | Black or African American | 2 | 1 | 3 |
| White | 18 | 14 | 32 | |
| Weight (kg) | Mean (SD) | 72.87 (13.88) | 75.73 (10.33) | 74.10 (12.40) |
| BMI (kg/m2) | Mean (SD) | 26.80 (3.15) | 27.55 (2.35) | 27.12 (2.82) |
Abbreviations: BMI, body mass index; N, number of participants; SD, standard deviation.
In total, 35 participants were randomly assigned to study sequences and received at least one dose of study drug. A single participant discontinued from Cohort 1 due to a family emergency. All other participants in Cohort 1 completed periods 1–4, and all participants in Cohort 2 completed periods 4–7, at which time the study was stopped following review of safety data.
Abemaciclib pharmacokinetics
After reaching C max, concentrations declined in a generally monophasic manner (Figure 2). Across abemaciclib doses of 200–600 mg, geometric mean t 1/2 values ranged from 21.6 to 27.6 h for abemaciclib. Median t max values ranged from 8 to 10 h for abemaciclib and for total analytes (where PK parameters were derived from the sum of the concentrations of abemaciclib, M2, and M20) (Tables S6–S8) across the abemaciclib dose range (Table 2). Assessment of dose proportionality demonstrated that the 95% CIs for AUC(0–t last), AUC(0–∞), and C max all contained 1.0, therefore proportionality can be statistically concluded over the dose range of 200–600 mg (Table S2). No dose‐dependent trend was observed in apparent total body clearance of abemaciclib.
FIGURE 2.
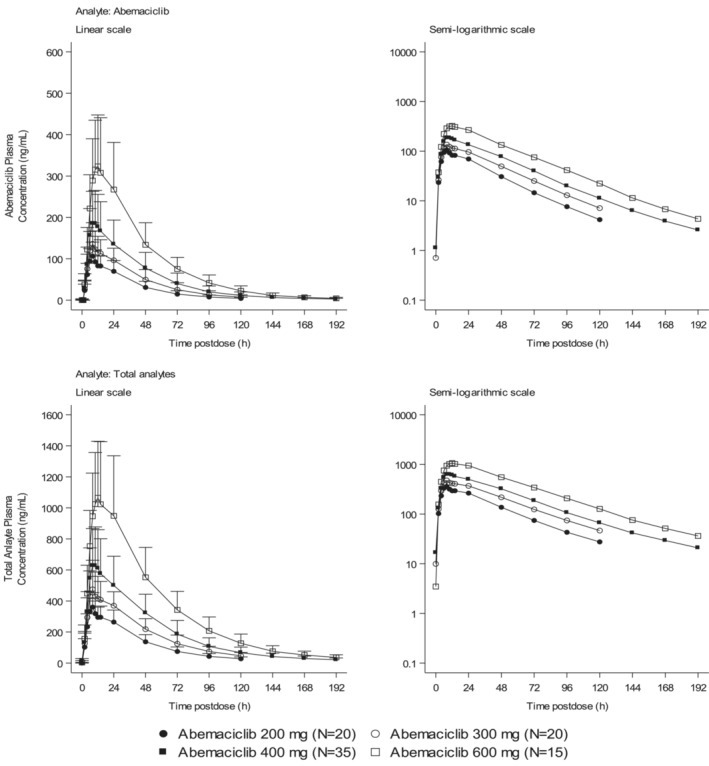
Arithmetic mean of plasma abemaciclib and total analyte (abemaciclib, M2, and M20) concentration versus time profiles following single oral doses of 200–600 mg abemaciclib in healthy participants. Values are ± standard deviation.
TABLE 2.
Summary of abemaciclib plasma pharmacokinetics.
| Geometric mean (%CV) | ||||
|---|---|---|---|---|
| Dose | Abemaciclib | Abemaciclib | Abemaciclib | Abemaciclib |
| 200 mg | 300 mg | 400 mg b | 600 mg | |
| N | 19 | 20 | 31 | 8 |
|
AUC (0–t last) (ng h/mL) |
3560 (38) | 5210 (37) | 7610 (46) | 14,600 (42) |
|
AUC (0–∞) (ng h/mL) |
3680 (38) | 5420 (40) | 7840 (47) | 14,800 (42) |
|
C max (ng/mL) |
102 (31) | 130 (40) | 182 (42) | 308 (45) |
|
t max a (h) |
8.23 (6.10–14.10) | 8.10 (6.10–24.10) | 10.05 (6.05–24.13) | 10.08 (10.05–14.07) |
|
t 1/2 (h) |
21.6 (14.2–30.0) | 22.6 (12.7–32.9) | 25.0 (14.8–36.6) | 27.6 (23.3–33.8) |
|
CL/F (L/h) |
54.4 (38) | 55.3 (40) | 51.0 (47) | 40.6 (42) |
|
Vz/F (L) |
1700 (31) | 1800 (28) | 1840 (40) | 1610 (47) |
|
V ss/F (L) |
1930 (33) | 2140 (33) | 2120 (41) | 1840 (48) |
Note: AUC (0–∞) = area under the concentration versus time curve from time zero to infinity; AUC (0–t last) = area under the concentration versus time curve from time zero to time t, where t is the last timepoint with a measurable concentration; %AUC (t last–∞) = percentage of AUC (0–∞) extrapolated; CL/F = apparent total body clearance of drug at steady state calculated after oral administration; C max = maximum observed drug concentration; CV = coefficient of variation; N = number of subjects; t 1/2 = half‐life associated with the terminal rate constant (λz) in non‐compartmental analysis; t max = time of maximum observed drug concentration; V ss/F = apparent volume of distribution at steady state after oral administration; V z/F = apparent volume of distribution during the terminal phase after oral administration..
Median (range).
Includes 400 mg abemaciclib data from Periods 4 and 5.
Effect of abemaciclib on HR and corrected QT interval
There were no significant changes in HR determined by central ECG analysis after 200, 300, 400, or 600 mg abemaciclib as the largest mean ΔΔHR did not exceed 10 bpm at any timepoint post‐dosing (Table S3). Therefore, the primary analysis of QT ER was based on ΔΔQTcF and not ΔΔQTbtb.
Categorical analyses of QTcF interval and PR showed 5 participants outside the defined ICH E14 guidance limits (Table 3): 2 of 34 participants showed a QTcF interval > 450 ms following a 400 mg dose of abemaciclib (1 participant had a mean QTcF value of 454 ms during Period 6 at 14‐h ECG collection, which is 14 ms above baseline and not clinically significant; another participant had a mean QTcF value of 452 ms at the 10 h post‐dose timepoint, 36 ms above baseline); 1 of 15 participants showed an increase in QTcF interval >30 ms following a dose of 600 mg abemaciclib. The elevated QTcF values were transient in nature, occurring during single timepoints, and were not considered clinically significant by the investigator.
TABLE 3.
Categorical analysis of QTc interval data.
| Dose | QTcF interval (ms) | Maximum increase in QTcF interval (ms) a | PR (ms) | QRS (ms) | |||
|---|---|---|---|---|---|---|---|
| >450 | >480 | >500 | >30 | >60 | >200 | >110 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Placebo (N = 34) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Abemaciclib 200 mg (N = 20) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Abemaciclib 300 mg (N = 20) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Abemaciclib 400 mg (N = 35) | 2 (5.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Abemaciclib 600 mg (N = 15) | 1 (6.7%) | 0 (0.0%) | 0 (0.0%) | 1 (6.7%) | 0 (0.0%) | 1 (6.7%) | 0 (0.0%) |
Note: n = number of participants; N = number of participants studied.
Baseline is defined as the mean of the pre‐dose measurements.
A single participant showed PR values >200 ms at the 2‐, 4‐, 8‐, 10‐, and 12‐h timepoints (ranging from 202 to 206 ms) following administration of 600 mg abemaciclib. These were similar to the participant's baseline value of 201 ms and were determined not to be clinically significant.
QT exposure–response analysis
ER analysis was conducted by pairing ECG data and PK data to delineate effects of abemaciclib and total analytes (abemaciclib, M2, and M20) plasma concentrations on QT interval. The upper bound of the 90% CI of the predicted ΔΔQTcF does not cross the 10 ms threshold at the highest observed abemaciclib and total analyte concentrations (Figure 3).
FIGURE 3.
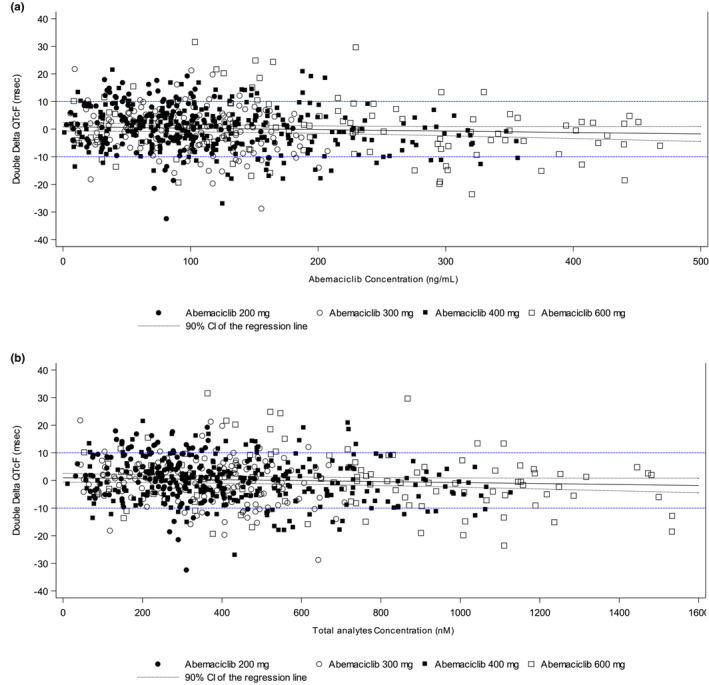
QTcF changes from baseline and placebo versus abemaciclib. (a) Plasma and (b) total analyte concentrations; dashed blue lines indicate upper and lower thresholds of the 90% confidence interval (CI).
The upper bound of the two‐sided 90% CI of the time‐matched ΔΔQTcF was below the 10 ms threshold at each of the eight timepoints evaluated over the 24‐h period, with the exception of 10 h post‐dose for the 600 mg dose, which showed an upper bound of 11 ms.
The slopes of ΔΔQTcF and abemaciclib, M2, M20, and total analyte concentrations were either nonsignificant, or significant but with a negative slope (p‐value > 0.05) (Table S4).
Safety and tolerability
Some 25% to 80% of participants administered abemaciclib reported adverse events (AEs), with the percentage increasing in a dose‐dependent manner. Of the participants reporting AEs, 80%–100% experienced investigator‐assessed drug‐related AEs, the majority of which (92%–100%) were mild (Common Terminology Criteria for Adverse Events [CTCAE] Grade 1) in severity, with the remainder categorized as moderate (CTCAE Grade 2), and five graded as severe (CTCAE Grade 3), after administration of 200 or 300 mg abemaciclib. The only drug‐related AE experienced by more than one participant was nausea or diarrhea. Drug‐related AEs experienced by more than one participant after administration of 400 or 600 mg abemaciclib were diarrhea, nausea, headache, vomiting, abdominal pain, and dyspepsia (Table S5). Two participants (Period 6–400 mg and Period 7–600 mg) received 2 mg loperamide during treatment for onset of diarrhea.
During Cohort 2, Period 4 (the DDI evaluation with loperamide), 5 severe AEs (SAEs) of elevated hepatic transaminases aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were experienced by 5 of 15 participants at the 24‐h timepoint post‐dose: 4 participants had been dosed with 8 mg loperamide + 400 mg abemaciclib, and 1 had been dosed with 8 mg loperamide + placebo.
The incidence of hepatic AEs were reviewed by the investigator and Safety Review Panel based on clinical judgment and protocol criteria. No confounding factors were identified, though all four of these participants had a history of cholecystectomy. There is no evidence historically of this being a factor in abemaciclib or loperamide absorption or toxicity. Analysis of PK data did not demonstrate evidence of outliers in relation to abemaciclib or loperamide exposure that might explain the increased hepatic transaminases in these four participants. Subsequent rechallenge of the four participants with 400 or 600 mg abemaciclib without loperamide resulted in no further AEs of hepatic transaminase elevation, and no clinically significant AEs, observed increases in AEs, or subsequent laboratory data abnormalities were noted with subsequent dosing.
Because both the frequency of mild gastrointestinal events had increased on 600 mg dose administration and three participants had moderate events of diarrhea which the investigator determined had significantly interfered with daily activities, the Safety Review Panel determined that these tolerability issues met the protocol‐specified criteria limiting further dose escalation. Thus, Period 8 was not completed, and no participants were administered a 900 mg dose. Therefore, 400 mg abemaciclib was determined to be the maximum tolerated dose (MTD) in this study.
No deaths or other SAEs occurred during this study, and no participants discontinued due to an AE.
DISCUSSION
The non‐antiarrhythmic drugs such as moxifloxacin 9 , 10 and ondansetron 11 cause a delay in cardiac repolarization, which can be measured physiologically as a delay in the QT interval on an ECG. A delay in cardiac repolarization is considered undesirable as it may lead to cardiac arrhythmia, such as torsade de pointes, leading to sudden cardiac death if patients are left untreated. 12 , 13 , 14 , 15
In vitro studies of abemaciclib and its major active metabolites M2 and M20 have not demonstrated blockade of the current produced by the human ether‐a‐go‐go‐related gene (hERG) potassium channel expressed in mammalian cells (unpublished data). This finding is also in line with the US Food and Drug Administration's recent S7B/E14 Q&A discussing the importance of in vitro hERG assays to determine risk assessment of delayed ventricular repolarization. 16 Importantly, no QTc prolongation was observed in dogs following single dose administration of abemaciclib up to 10 mg/kg (replicating the exposure observed in humans, based upon C max following a single 200 mg dose). 17 In this dose‐escalation Phase 1 study, single ascending oral doses of 200, 300, 400, or 600 mg abemaciclib or placebo were administered to healthy participants to determine the relationship between plasma concentrations of abemaciclib, its major active metabolites M2 and M20, and QT interval.
At the highest feasible single dose level evaluated in this study (600 mg), the geometric mean C max for abemaciclib was 308 ng/mL (45% coefficient of variation [CV]), which was similar to that achieved at steady state by the approved starting dose in advanced or metastatic breast cancer in MONARCH 1 (305 ng/mL [37% CV]), MONARCH 2 (258 ng/mL [38% CV]), and MONARCH 3 (249 ng/mL [42% CV]). 18 , 19 , 20 , 21
Given that abemaciclib is a substrate of CYP3A4 and strong inhibitors of CYP3A4 increase abemaciclib exposures significantly, 22 higher doses up to 900 mg were planned, to yield exposures similar to those expected when abemaciclib is coadministered with CYP3A inhibitors. The 900 mg dose level could not be evaluated due to dose‐limiting toxicities (DLTs) observed at the 600 mg dose level in this study. Although the supratherapeutic exposures could not be achieved in healthy participants, we have demonstrated that abemaciclib had no statistically or clinically significant effect on QT at any dose level (200–600 mg).
The results of the primary ER analysis did not reveal a prolongation of the mean ΔΔQTcF interval at any of the timepoints studied. Abemaciclib therefore does not prolong the QT interval to any clinically relevant extent. There was no significant impact on ΔΔHR (with no observed changes >10 bpm), thus the primary analysis was based solely on QTcF. Participation in the study was not limited to one sex; however, 80% of the participants enrolled overall in the study were female. No sex‐related differences in systemic exposure have been observed in non‐clinical studies of abemaciclib, or in population PK analyses in cancer patients and healthy participants. 18 , 23 The responses of regulators to E14 guidance suggest that variability of baseline demographic parameters (beyond those which affect exposure, e.g., BMI/weight) should not introduce a large difference in QT response to a drug. 24 In accordance with regulatory guidance, no sex‐specific subgroup analyses were performed as a positive signal for QTc prolongation was not demonstrated.
Single 200, 300, and 400 mg doses of abemaciclib were well tolerated by healthy participants, with a low incidence of treatment‐related AEs (primarily diarrhea and nausea), the majority were reported as mild (CTCAE Grade 1) in severity. However, the frequency and severity of treatment‐related gastrointestinal events (primarily diarrhea, nausea, and vomiting) increased upon a single 600 mg dose administration, which significantly interfered with daily activities, and the dose escalation was stopped based on this evidence of dose limiting toxicities. Period 8 was not initiated, and the 900 mg dose level was not tested. Therefore, the MTD for a single oral dose of abemaciclib in healthy participants was identified as 400 mg.
In conclusion, a single abemaciclib dose up to 400 mg had no statistically or clinically relevant effect on QTc, HR, PR, or QRS intervals.
AUTHOR CONTRIBUTIONS
J.C.C., A.Y.C., J.R., H.C., P.K., and P.K.T. wrote the manuscript; J.C.C., A.Y.C., J.R., H.C., P.K., and K.T. designed the research; J.C.C., A.Y.C., J.R., H.C., P.K., and P.K.T. performed the research; J.C.C., A.Y.C., J.R., H.C., P.K., and P.K.T. analyzed the data.
FUNDING INFORMATION
The research described in this article was funded by Eli Lilly and Company. Covance Inc. were responsible for the clinical conduct of the study and bioanalytical analyses described in this article.
CONFLICT OF INTEREST STATEMENT
J.C.C., P.K.T., A.Y.C., and P.K. are current or former employees of Eli Lilly and Company. H.C. and J.R. are current or former employees of LabCorp Drug Development (formerly Covance) and responsible for the study conduct. The study was funded by Eli Lilly and Company. All the other authors declared no competing interests for this work.
Supporting information
Figure S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
ACKNOWLEDGMENTS
The authors thank the study participants, investigators, and site staff for their participation. Writing and editing was provided by Nicholas Pulliam, an employee of Eli Lilly and Company, further writing by Ndidi Uzor, an employee of Syneos Health, and further editing by Dana Schamberger, also an employee of Syneos Health. Covance Inc. was responsible for study conduct and bioanalytical analyses.
Chappell JC, Chiang AY, Royalty J, Coleman H, Kulanthaivel P, Turner PK. Abemaciclib does not increase the corrected QT interval in healthy participants. Clin Transl Sci. 2023;16:1617‐1627. doi: 10.1111/cts.13573
REFERENCES
- 1. Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in‐vivo cell cycle‐dependent/independent anti‐tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darpo B, Benson C, Dota C, et al. Results from the IQ‐CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin Pharmacol Ther. 2015;97:326‐335. [DOI] [PubMed] [Google Scholar]
- 3. Darpo B, Sarapa N, Garnett C, et al. The IQ‐CSRC prospective clinical phase 1 study: "can early QT assessment using exposure response analysis replace the thorough QT study?". Ann Noninvasive Electrocardiol. 2014;19:70‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Darpo B, Garnett C, Keirns J, Stockbridge N. Implications of the IQ‐CSRC prospective study: time to revise ICH E14. Drug Saf. 2015;38:773‐780. [DOI] [PubMed] [Google Scholar]
- 5. International Conference on Harmonisation (ICH) . Final concept paper: E14 Q&As (R3): revision of ICH E14 Q&As (R2). International Conference on Harmonisation. 2015. https://database.ich.org/sites/default/files/E14_Q%26As_R3_Concept_Paper.pdf
- 6. Garnett C, Bonate PL, Dang Q, et al. Scientific white paper on concentration‐QTc modeling. J Pharmacokinet Pharmacodyn. 2018;45:383‐397. [DOI] [PubMed] [Google Scholar]
- 7. Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non‐small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6:740‐753. [DOI] [PubMed] [Google Scholar]
- 8. Turner PK, Chappell JC, Chiang AY, et al. P‐gp inhibition by abemaciclib: a two‐way drug‐drug interaction study with loperamide in healthy subjects. American Society for Clinical Pharmacology and Therapeutics 2018 Annual Meeting. Journal of Clinical Oncology; 2018:S5‐S97. [Google Scholar]
- 9. Smith BP, Vandenhende FR, DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17:1278‐1283. [DOI] [PubMed] [Google Scholar]
- 10. Bottcher M, Düngen H‐D, Corcea V, et al. Vericiguat: a randomized, phase Ib, placebo‐controlled, double‐blind, QTc interval study in patients with chronic coronary syndromes. Am J Cardiovasc Drugs. 2023;23:145‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taubel J, Ferber G, Lorch U, Batchvarov V, Savelieva I, Camm AJ. Thorough QT study of the effect of oral moxifloxacin on QTc interval in the fed and fasted state in healthy Japanese and Caucasian subjects. Br J Clin Pharmacol. 2014;77:170‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl. 2001;3:K70‐K80. [Google Scholar]
- 13. Hafermann MJ, Namdar R, Seibold GE, Page RL 2nd. Effect of intravenous ondansetron on QT interval prolongation in patients with cardiovascular disease and additional risk factors for torsades: a prospective, observational study. Drug Healthc Patient Saf. 2011;3:53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nachimuthu S, Assar MD, Schussler JM. Drug‐induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3:241‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Straus SMJM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362‐367. [DOI] [PubMed] [Google Scholar]
- 16. Food and Drug Administration, HHS . E14 and S7B Clinical and Nonclinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential‐‐Questions and Answers: Guidance for Industry. 2022. https://www.fda.gov/media/161198/download
- 17. Posada MM, Morse BL, Turner PK, Kulanthaivel P, Hall SD, Dickinson GL. Predicting clinical effects of CYP3A4 modulators on abemaciclib and active metabolites exposure using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2020;60:915‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chigutsa E, Kambhampati SRP, Karen Sykes A, Posada MM, van der Walt J, Turner PK. Development and application of a mechanistic population modeling approach to describe abemaciclib pharmacokinetics. CPT Pharmacometrics Syst Pharmacol. 2020;9:523‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin Cancer Res. 2017;23:5218‐5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with Fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875‐2884. [DOI] [PubMed] [Google Scholar]
- 21. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced. Breast Cancer. 2017;35:3638‐3646. [DOI] [PubMed] [Google Scholar]
- 22. Eli Lilly and Company . Verzenio (Abemaciclib) [package insert]. 2023.
- 23. Tate SC, Sykes AK, Kulanthaivel P, Chan EM, Turner PK, Cronier DM. A population pharmacokinetic and pharmacodynamic analysis of abemaciclib in a phase I clinical trial in cancer patients. Clin Pharmacokinet. 2018;57:335‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Food and Drug Administration, HHS . International Conference on Harmonisation; guidance on E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs; availability. Notice. Fed Regist. 2005;70:61134‐61135. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8


