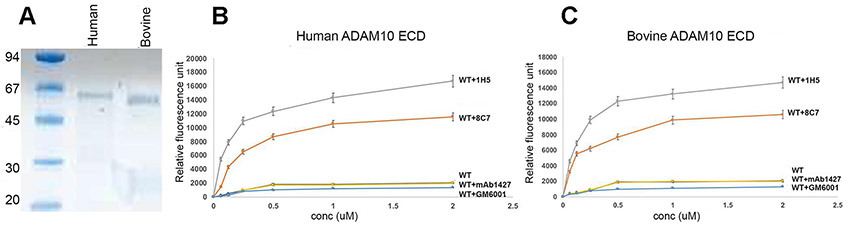
Fig. 7.
(A) SDS-PAGE profile of purified human (h) and bovine (b) catalytically active ADAM10 extracellular domains (ECD). (B and C) FRET-based peptide cleavage assays. The data shows that 1H5, similar 8C7 (but even more efficiently), promotes cleavage of a short peptide substrate by ADAM10. The data represent mean of triplicate determinations and two independent experiments (mean fluorescence ± SEM). Maximum dispersion was within 10 % of the mean value. Bovine or human ADAM10(ECD)-antibody complexes were formed at 1:1 molar ratio prior to the assay, and the assay was carried out in the presence of 50 μM of a fluorogenic peptide as described in the Materials and Methods section. GM6001 is a MMP inhibitor, which was also used as a control. Statistical analysis revealed significant difference/increase in peptide cleavage (fluorescence) between the 1H5 and 8C7 treated and untreated ADAM10 samples (p < 0.001, for both 1H5 and 8C7 treatment, one way ANOVA with a Dunnett’s multiple comparison post hoc test), as well as between the 1H5-treated and 8C7-treated ADAM10 samples (p = 0.003, two-tailed independent t test). Treatment with the control mAb1427 or the GM6001 inhibitor did not significantly change the ADAM10 enzymatic activity (p > 0.05).