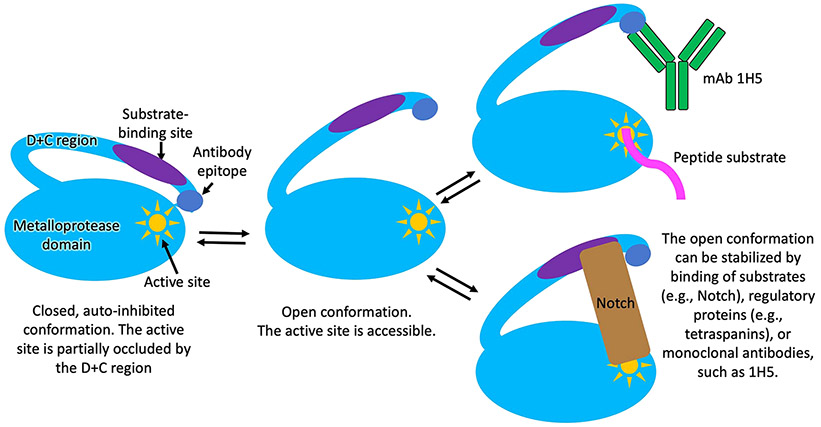
Fig. 8.
Schematic representation of a proposed mechanism for ADAM10 activation and interactions with substrates and the 1H5 antibody. In the autoinhibited conformation, which is the predominant conformation of the ADAM10 ectodomain observed in solution [31], the cysteine rich domain partially occludes the ADAM10 active site, hindering substrate binding. In the open ADAM10 conformation, the active site is fully accessible for substrate binding [39]. In addition to interacting with the active site, ADAM10 cell-surface substrates also interact with the D+C region of the molecule, which is required for substrate selection and/or proper substrate positioning for productive cleavage. The open ADAM10 conformation can be stabilized by binding of substrates, such as Notch, or other regulatory molecules, such as tetraspanins. The latter not only regulate the activity of ADAM10 by stabilizing the open conformation, but also selectively enhance the cleavage of certain substrate, while downregulating the cleavage of other substrates [2,3,14,31,39]. The 1H5 mAb, in this regard, acts in a similar fashion to the tetraspannins: it (i) selectively binds and stabilizes the activated, open ADAM10 conformation; and (ii) it selectively downregulates the cleavage of certain ADAM10 substates (Notch), while upregulating (or not affecting) the cleavage of other substrates (e.g., peptides, APP).