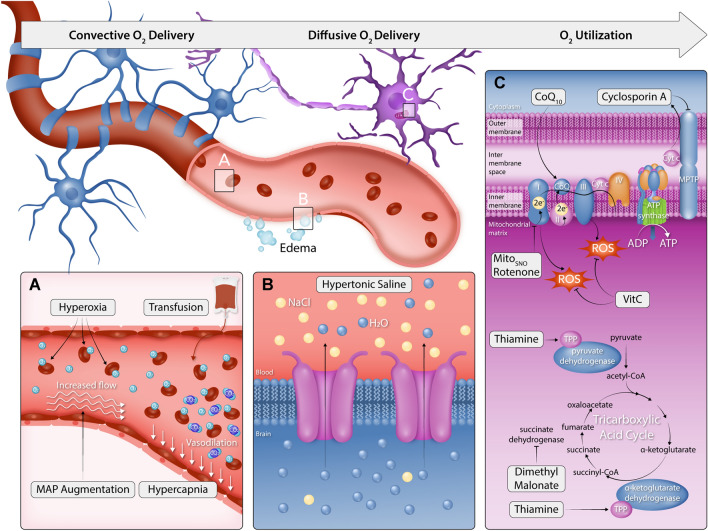
Fig. 3.
Therapies for hypoxic–ischemic brain injury in the context of the oxygen cascade. This figure illustrates the dysfunction of the oxygen cascade in hypoxic–ischaemic brain injury and the targeted therapies aimed at improving oxygen transport to the brain, brain oxygenation, and/or oxygen utilisation (mitochondrial function). A To therapeutically target convection oxygen delivery, MAP augmentation and hypercapnia aim to increase cerebral perfusion by increasing the hydraulic pressure head for flow and lower cerebral vascular resistance through CO2-mediated cerebral vasodilation, respectively. Conversely, hyperoxia and transfusion aim to increase oxygen content by increasing the pressure of dissolved O2 and haemoglobin concentration, respectively. B To therapeutically target diffusion limitations, hypertonic saline has been shown to reduce cerebral oedema as well as the oxygen gradient between cerebral venous blood and parenchyma, indicating improved oxygen diffusion. C Oxygen utilisation and mitochondrial dysfunction impairments have been well documented in HIBI and global ischemic brain disease models. Complex 1 generates excess ROS that leads to the dysfunction of key enzymes and metabolic processes within the TCA cycle. Further, increased calcium leads to mitochondrial efflux of cytochrome C and pro-apoptotic signalling. Experimental models have used dimethylmalonate to block excessive post-ischaemia oxidation of succinate, whilst MitoSNO and Rotenone have been used to selectively block the downstream ROS production by complex 1 following reverse electron transport. Cyclosporin A has been administered to inhibit the mitochondrial permeability transition pore and reduce cytochrome C's efflux and consequent apoptotic signalling. Finally, the co-factors thiamine and co-enzyme Q10 (Co-Q10) have been administered to restore metabolic function following ischaemia–reperfusion injury