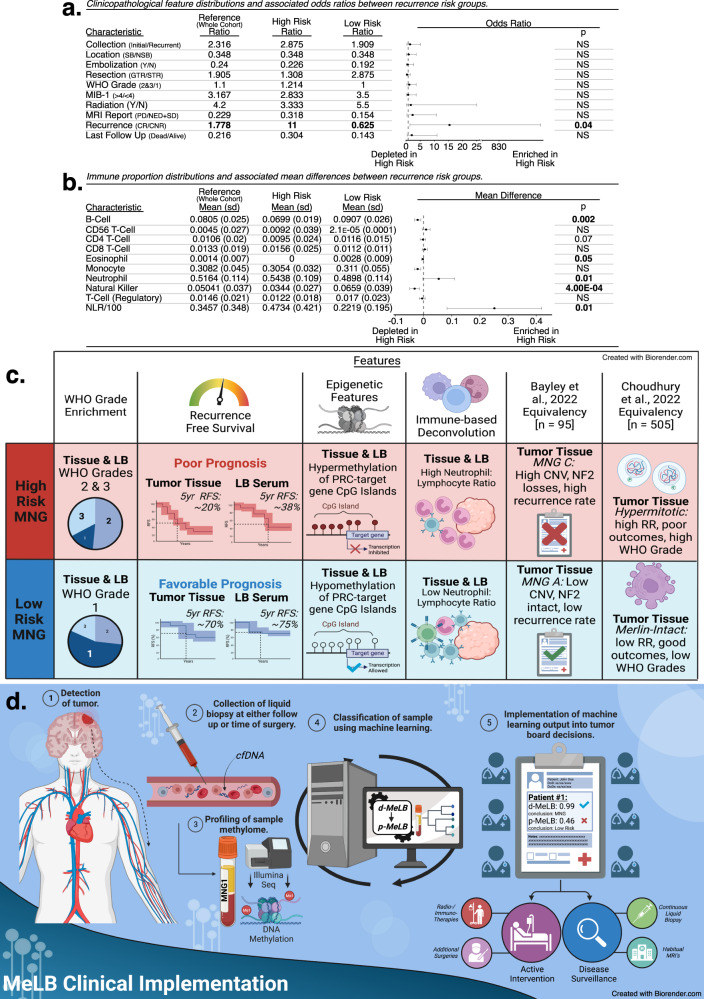
Fig. 3. Clinicopathological and molecular characterization of serum from patients with meningioma predicted to present distinct recurrence risk outcomes through p-MeLB.
a Clinicopathological feature proportions and associated odds ratios (p-values: two-sided Fisher’s Exact test; error bars: 95% confidence interval estimates) derived from the comparison between meningioma serum samples predicted to present high or low recurrence risks. Reference column depicts the mean proportion of each feature across the whole cohort. SB Skull-base, NSB Non-Skull Base, Y Yes, N No, GTR Gross Total Resection, STR Subtotal Resection, PD Progressive Disease, SD Stable Disease, NED Non-Enhancing Disease, CR Confirmed Recurrence, CNR Confirmed No Recurrence; Bolded features are those with observed statistical significance. b Immune cell proportions and associated mean differences derived from the comparison between meningioma serum samples predicted to present high or low recurrence risks (error bars: mean difference 95% confidence interval; p-values: two-sided t-test). Reference column depicts the mean proportion across the whole cohort. NLR Neutrophil-Lymphocyte Ratio. Bolded features are those with observed statistical significance. c Schematic summarization of observed clinicopathological and molecular features across samples (LB-serum and/or tissue) from patients with meningiomas predicted to present high or low risk of recurrence through p-MeLB. LB liquid biopsy, RFS Recurrence Free Survival, RR Recurrence Risk, MNG C Bayley Meningioma C group, CNV copy number variation, RR recurrence risk, PRC Polycomb Repressive Complex. d Schematic representation—clinical application of liquid biopsy DNA methylation-based diagnostic and prognostic classifiers in patients suspected to present meningioma. MeLB Meningioma epigenetic Liquid Biopsy, cfDNA cell-free DNA, d-MeLB and p-MeLB diagnostic- and prognostic- Meningioma Epigenetic Liquid Biopsy, respectively, MRI magnetic resonance imaging.