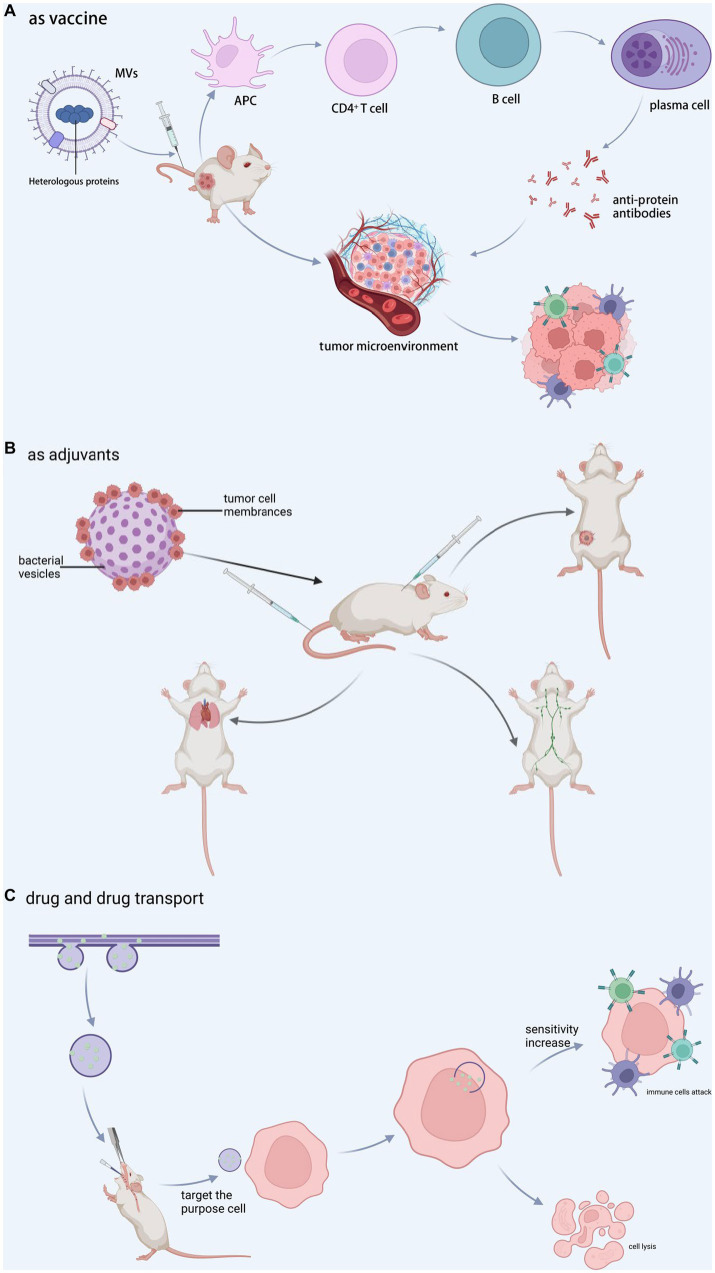
Figure 4.
MVs in clinical applications. (A) Vaccine-associated proteins are encapsulated during vesicle formation, and the complex is administered into tumor-bearing animals, resulting in a decrease in tumor cells and an increase in CTL cells via immunomodulation or direct interaction with tumor tissue. (B) Tumor cell membranes and bacterial vesicles are combined to generate new vesicles, which are then used as adjuvants in customized immunotherapy, inguinal lymph node activation, lung metastasis inhibition, and recurrence inhibition. (C) The medication penetrates during vesicle formation and afterwards targets the target cells, increasing cell sensitivity and encouraging target cell attack and cell destruction by immune cells. BioRender.com was used to create this.