Abstract
Cue-induced reward-seeking behaviors are regulated by both the affective and cognitive control systems of the brain. This study aimed at investigating how individual differences in affective and cognitive responses to cues predicting food rewards contribute to the regulation of cue-induced eating. We recorded electroencephalogram (EEG) from 59 adults while they viewed emotional and food-related images that preceded the delivery of food rewards (candies) or non-food objects (beads). We measured the amplitude of the late positive potential (LPP) in response to a variety of motivationally relevant images and power in the theta (4–8 Hz) frequency band after candies or beads were dispensed to the participants. We found that individuals with larger LPP responses to food images than to pleasant images (C>P group) ate significantly more during the experiment than those with the opposite response pattern (P>C group, p < 0.001). Furthermore, we found that individuals with higher theta power after dispensation of the candy than of the bead (θCA>θBE) ate significantly more than those with the opposite response pattern (θBE>θCA, p < 0.001). Finally, we found that the crossed P>C and θBE>θCA group ate less (p < 0.001) than did the other three groups formed by crossing the LPP and theta group assignments, who exhibited similar eating behavior on average (p = 0.662). These findings demonstrate that individual differences in both affective and cognitive responses to reward-related cues underlie vulnerability to cue-induced behaviors, underscoring the need for individualized treatments to mitigate maladaptive behaviors.
Keywords: Motivational salience, Cognitive control, Late positive potential, Theta band, Event-related potentials, Time-frequency analysis
1. Introduction
Overweight and obesity, characterized by a body mass index (BMI) of at least 25 kg/m2 and at least 30 kg/m2, respectively, increase the risk of cardiovascular disease, diabetes, and several types of cancer [1]. Losing even a modest amount of weight can have substantial health benefits, but most weight-loss interventions yield short-lived, suboptimal results [2,3]. Identifying the neurobiological mechanisms underlying excessive eating, the ultimate cause of weight gain [4,5], can help clinicians target the root causes of overeating, personalize interventions for weight loss, and improve weight loss treatment outcomes.
Neurobiological models of obesity posit that both the brain’s reward and cognitive control systems play a major role in regulating food intake [6–8]. The reward system guides eating behavior with “bottom-up” signals that dynamically assign motivational salience to food rewards and the cues associated with them [9,10]. In contrast, cognitive control systems exert “top-down” control over eating behavior by enabling the implementation of intentional, goal-directed behaviors [11]. Failure of either mechanism can lead to maladaptive eating patterns, overconsumption of hyper-palatable foods, and weight gain [8].
Preclinical findings have demonstrated that animals differ significantly in their predisposition to display bottom-up versus top-down driven behaviors in the presence of reward-related cues [12]. For example, after rats are repeatedly presented with discrete cues predicting food rewards, rodents develop Pavlovian conditioned approach. However, some animals, called “sign-trackers,” will approach the cue, while others, called “goal-trackers,” will approach the location where the food reward is delivered [13]. These behavioral differences are the consequence of different cognitive-motivational styles, in which either bottom-up or top-down processes dominate [12]. Animals classified as sign-trackers assign high motivational salience to the cues, which makes these cues attractive and allows them to effectively trigger compulsive, reward-seeking behaviors that are often resistant to devaluation and extinction [14,15]. In contrast, goal-trackers do not attribute incentive salience to reward-related cues and, thanks to better top-down attentional control, are less prone to cue-induced compulsive reward-seeking behaviors.
Because humans are also known to exhibit sign-tracking behaviors [16,17], we hypothesized that individuals who attribute high motivational significance to reward-related cues would be more susceptible to cue-induced eating [18,19]. To test our hypothesis, we developed an assessment procedure called the “cued food delivery task” [20]. This task differs from standard experimental paradigms used to assess cue reactivity because it allows researchers to assess a participant’s responses to a broad range of motivationally relevant stimuli while also providing participants the opportunity to immediately consume food rewards dispensed during the task. During this task, participants view emotional, neutral, and food-related images. After a food-related image is presented on the screen, the participant is dispensed a chocolate candy, which they may choose to either eat or discard [20]. By recording electroencephalogram (EEG) throughout the task, we were able to compute the amplitude of the late positive potential (LPP) in response to a broad range of images.
The LPP is a component of the event-related potentials (ERPs) that reliably tracks motivational relevance. Typically, highly salient images such as erotica and mutilations prompt larger LPP responses compared to images with lower salience, such as romantic or sad images [21–25]. Prior research also showed that the affective modulation of the LPP has high internal and test-retest reliability [26–28,23] and is robust to manipulations of the images’ perceptual characteristics [29,30], making it an excellent tool to assess how individual differences in affective reactivity might influence behaviors [25,31]. By applying cluster analysis, a multivariate classification technique [32], to the LPP responses evoked by the range of stimuli presented during the cued food delivery task, we identified two reactivity profiles associated with individual differences in the tendency to attribute motivational salience to reward-related cues. Individuals in one profile showed higher LPP responses to food-related cues than pleasant stimuli (C>P), while individuals in the other profile showed higher LPP responses to pleasant stimuli than to food-related cues (P>C). Importantly, individuals classified as C>P ate significantly more during the experiment than those classified as P>C [33].
While these findings support the hypothesis that attributing higher motivational salience to food-related cues than to pleasant non-food-related stimuli increases vulnerability to cue-induced eating, they are silent about the role that individual differences in the engagement of top-down cognitive control systems have in regulating cue-induced eating.
Several ERP components have been used to assess executive functions, action monitoring, and cognitive control, including the N2, the error-related negativity, and the feedback-related negativity [34–38]. However, results from time-frequency analyses suggest that these ERP components reflect a common underlying feature: power increases in the EEG theta band (4–8 Hz) [39]. Because theta power over midfrontal scalp sites increases when participants try to inhibit prepotent responses [40,41] or perform difficult tasks [42], activity in the theta frequency band has been proposed as a reliable correlate of the engagement of top-down cognitive control mechanisms [39,43]. Therefore, we hypothesized that measuring changes in theta power during food-related and non-food-related decision-making in the cued food delivery task would provide us with a window to monitor the engagement of cognitive control systems during the task.
The present study aimed to investigate the role of individual differences in both the attribution of motivational salience to food-related cues and the engagement of cognitive control systems in regulating cue-induced eating during the cued food delivery task. First, we expected to replicate our previous findings showing that a) clustering individuals using LPP responses evoked by food-related and non-food-related images yields two groups, one with larger responses to the food-related cues than to the pleasant stimuli (C>P) and one with the opposite reactivity profile (P>C), and b) that individuals with the C>P profile would eat more than individuals with the P>C profile. Then, we aimed to elucidate the extent to which engagement of cognitive control systems—as indexed by theta power—differs between P>C and C>P individuals. Observing differences in midfrontal theta power between the C>P and P>C groups would suggest that, in line with animal models [12,44–46], individuals that attribute high motivational salience to food-related cues also have poor top-down control over cue-induced behaviors. Alternatively, results demonstrating that individual differences in midfrontal theta power predict eating behavior regardless of C>P and P>C status would suggest that top-down cognitive control and bottom-up motivational processes independently contribute to the regulation of cue-induced eating in humans.
By clarifying whether motivational salience and cognitive control mechanisms interact to regulate cue-induced eating or do so independently, we may inform clinical researchers of effective mechanistic targets for weight loss and other clinical interventions aimed at reducing maladaptive, reward-seeking behaviors.
2. Materials and methods
2.1. Participants
Using flyers and magazine and newspaper advertisements, we recruited sixty research participants from the Houston, TX, metro area. Participants were eligible if they were 18 to 65 years of age, were neither pregnant nor breastfeeding, and did not have a history of psychiatric disorders, seizures, head injuries with loss of consciousness, uncorrected visual impairments, eating disorders, allergies, or any other illnesses that would prevent them from eating chocolate candy. Participants received monetary compensation for their time and travel totaling up to $60 each. One participant was excluded from the final analysis due to incomplete data. Tables 1 and 2 show the demographic information for the participant sample.
Table 1.
Demographic, biometric, and self-reported data for all subjects and the LPP and θ-based participant groups.
| Mean (SD) | Mean (SD) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | All subjects(n = 59) | C>P(n = 28) | P>C(n = 31) | p(C>Pvs.P>C) | θCA>θBE(n = 22) | θBE>θCA(n = 37) | p(θCA>θBE vs. θBE>θCA) |
|
| |||||||
| Age, years | 45 (11.67) | 43 (14.09) | 48 (8.52) | 0.10 | 43 (11.61) | 47 (11.62) | 0.21 |
| Women, % | 46 | 39 | 52 | 0.34 | 82 | 24 | 0.56 |
| Race, % | |||||||
| Black/African American | 64 | 75 | 55 | 73 | 59 | ||
| White/Caucasian | 24 | 14 | 32 | 18 | 27 | ||
| Asian | 7 | 11 | 3 | 5 | 14 | ||
| More than one race | 3 | 0 | 6 | 5 | 3 | ||
| I prefer not to say | 2 | 0 | 3 | 0 | 3 | ||
| BMI, kg/m2 | 31 (7.75) | 30 (8.19) | 31 (7.43) | 0.69 | 31 (0.92) | 31 (7.42) | 0.85 |
| Hispanic or Latino ethnicity, % | 12 | 18 | 6 | 14 | 11 | ||
| BIS attention | 15.57 (3.63) | 15.52 (4.10) | 15.61 (3.23) | 0.92 | 15.62 (2.40) | 15.54 (4.20) | 0.94 |
| BIS motor | 21.26 (3.96) | 21.33 (4.27) | 21.19 (3.74) | 0.89 | 21.62 (4.20) | 21.05 (3.86) | 0.61 |
| BIS Non-planning | 13.22 (2.63) | 13.04 (2.68) | 13.39 (2.62) | 0.62 | 12.57 (2.20) | 13.59 (2.80) | 0.16 |
| Center for Epidemiologic Studies Depression Scale | 8.53 (4.76) | 8.85 (5.44) | 8.26 (4.15) | 0.64 | 7.81 (3.76) | 8.95 (5.24) | 0.39 |
| Snaith-Hamilton Pleasure Scale | 1.14 (2.54) | 1.33 (2.99) | 0.97 (2.12) | 0.59 | 1.05 (3.04) | 1.19 (2.26) | 0.84 |
| PANAS (+) | 33.28 (9.59) | 31.15 (10.08) | 35.13 (8.89) | 0.12 | 33.90 (8.74) | 32.92 (10.14) | 0.71 |
| PANAS (−) | 17.62 (7.64) | 18.19 (7.24) | 17.13 (8.05) | 0.60 | 16.95 (8.37) | 18.00 (7.28) | 0.62 |
| Power of Food Scale | 51.88 (19.22) | 48.70 (19.88) | 54.65 (18.50) | 0.24 | 50.00 (21.7) | 52.95 (17.87) | 0.58 |
| Food Cravings Questionnaire | 101.69 (36.56) | 99.07 (37.65) | 103.97 (36.05) | 0.62 | 96.62 (37.19) | 104.57 (36.40) | 0.43 |
| WREQ routine restraint | 1.64 (0.87) | 1.43 (0.58) | 1.83 (1.04) | 0.08 | 1.46 (0.69) | 1.75 (0.95) | 0.23 |
| WREQ compensatory restraint | 2.17 (0.84) | 2.05 (0.81) | 2.27 (0.85) | 0.32 | 2.17 (0.92) | 2.16 (0.80) | 0.96 |
| WREQ susceptibility to external cues | 2.30 (1.00) | 2.21 (1.06) | 2.37 (0.96) | 0.57 | 2.41 (1.15) | 2.23 (0.92) | 0.52 |
| WREQ emotional eating | 2.09 (1.02) | 1.95 (1.18) | 2.21 (0.86) | 0.34 | 2.13 (1.15) | 2.06 (0.95) | 0.79 |
| SLIM | 0.72 (39.15) | −3.24 (33.45) | 4.30 (43.92) | 0.46 | −11.70 (36.43) | 8.11 (39.31) | 0.06 |
| Number of candies eaten | 11 (17.19) | 14 (20.02) | 8 (13.94) | 0.21 | 14 (19.66) | 9 (15.42) | 0.22 |
Table 2.
Demographic, biometric, and self-reported data for crossed participant groups
| Mean (SD) | p (P>C & θBE>θCA vs P>C & θCA>θBE) | Mean (SD) | p (P>C & θBE>θCA vs. C>P & θBE>θCA) | Mean (SD) | p (P>C & θBE>θCA vs. C>P & θCA>θBE) | ||
|---|---|---|---|---|---|---|---|
| Characteristic | P>C & θBE>θCA (n = 20) | P>C & θCA>θBE (n = 11) | C>P & θBE>θCA (n = 17) | C>P & θCA>θBE (n = 11) | |||
|
| |||||||
| Age, years | 47 (9.15) | 48 (7.62) | 0.77 | 46 (14.26) | 0.76 | 37 (12.63) | 0.02 |
| Women, % | 50 | 55 | 0.81 | 47 | 0.86 | 27 | 0.22 |
| Race, % | |||||||
| Black/African American | 50 | 64 | 71 | 82 | |||
| White/Caucasian | 35 | 27 | 18 | 9 | |||
| Asian | 5 | 0 | 12 | 9 | |||
| More than one race | 5 | 9 | 0 | 0 | |||
| I prefer not to say | 5 | 0 | 0 | 0% | |||
| BMI, kg/m2 | 30 (5.73) | 33 (9.83) | 0.28 | 32 (9.11) | 0.46 | 28 (6.15) | 0.34 |
| Hispanic or Latino ethnicity, % | 10 | 27 | 12 | 0 | |||
| BIS attention | 15.20 (3.38) | 16.36 (2.94) | 0.35 | 15.94 (5.08) | 0.60 | 14.80 (1.32) | 0.72 |
| BIS motor | 21.20 (3.07) | 21.18 (4.90) | 0.99 | 20.88 (4.72) | 0.81 | 22.10 (3.48) | 0.47 |
| BIS Non-planning | 13.65 (2.85) | 12.91 (2.17) | 0.46 | 13.53 (2.83) | 0.90 | 12.20 (2.30) | 0.17 |
| Center for Epidemiologic Studies Depression Scale | 8.05 (4.02) | 8.64 (4.54) | 0.71 | 10.00 (6.36) | 0.27 | 6.90 (2.60) | 0.42 |
| Snaith-Hamilton Pleasure Scale | 1.45 (2.52) | 0.09 (0.30) | 0.09 | 0.88 (1.93) | 0.45 | 2.10 (4.25) | 0.60 |
| PANAS (+) | 34.95 (9.60) | 35.45 (7.85) | 0.88 | 30.53 (10.52) | 0.19 | 32.20 (9.75) | 0.47 |
| PANAS (−) | 16.25 (6.26) | 18.73 (10.75) | 0.42 | 20.06 (8.02) | 0.11 | 15.00 (4.37) | 0.58 |
| Power of Food Scale | 54.45 (17.61) | 55.00 (20.90) | 0.94 | 51.18 (18.56) | 0.59 | 44.50 (22.32) | 0.19 |
| Food Cravings Questionnaire | 105.05 (37.51) | 102.00 (34.93) | 0.83 | 104.00 (36.19) | 0.93 | 90.70 (40.54) | 0.34 |
| WREQ routine restraint | 1.98 (1.13) | 1.55 (0.82) | 0.27 | 1.47 (0.61) | 0.10 | 1.37 (0.53) | 0.12 |
| WREQ compensatory restraint | 2.28 (0.81) | 2.24 (0.97) | 0.90 | 2.02 (0.79) | 0.32 | 2.10 (0.90) | 0.58 |
| WREQ susceptibility to external cues | 2.25 (0.83) | 2.58 (1.18) | 0.37 | 2.21 (1.05) | 0.90 | 2.22 (1.14) | 0.94 |
| WREQ emotional eating | 2.12 (0.73) | 2.36 (1.07) | 0.46 | 1.99 (1.18) | 0.68 | 1.88 (1.24) | 0.51 |
| SLIM | 17.95 (39.16) | −20.50 (42.70) | 0.02 | −3.45 (37.31) | 0.10 | −2.91 (28.17) | 0.13 |
| Number of candies eaten | 4 (8.89) | 15 (18.76) | 0.04 | 14 (19.74) | 0.06 | 14 (21.42) | 0.09 |
2.2. Study procedures
The study consisted of an eligibility screening of potential participants via telephone followed by an in-person laboratory visit. A research assistant met with the participant at each laboratory visit to explain the study and obtain informed consent. The research assistant then collected the participant’s biometric information, including height and weight, and then administered a series of computerized questionnaires. After completing the questionnaires, the research assistant placed an EEG net on the participant’s head and instructed the participant on how to complete the cued food delivery task. The research assistant then left the room and began both the EEG recording and the cued food delivery task. After completion of the EEG session and task, the participant was debriefed and given financial compensation. All study procedures were approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
2.3. Questionnaires
Our computerized questionnaires consisted of those assessing hunger and satiety, eating habits, impulsivity, mood, affect, and hedonic tone. To assess hunger and satiety, we administered the Satiety Labeled Intensity Magnitude (SLIM) scale [47] to each participant before and after the completion of the cued food delivery task. To ascertain eating habits, we used the weight-related eating questionnaire (WREQ) [48], the Power of Food Scale (PFS) [49], and the Food Cravings Questionnaire (FCQ) [50], which measure variables such as susceptibility to external cues, the influence of a food-abundant environment on eating, and food cravings, respectively. To measure impulsivity, we administered the Barratt Impulsiveness Scale (BIS) [51]. Finally, to identify variables relating to affect, mood, and hedonic tone, we administered the Positive and Negative Affect Schedule (PANAS) [52], the Center for Epidemiologic Studies Depression (CESD) Scale [53], and the Snaith-Hamilton Pleasure Scale (SHAPS) [54], respectively.
2.4. Cued food delivery task
Participants completed the cued food delivery task depicted in Fig. 1 [20] with the addition of a control condition in which they were also dispensed plastic beads. During the task, participants viewed emotional, neutral, and food-related images presented on a 17-inch computer screen using E-Prime software (version 2.0.8.74; Psychology Software Tools, Inc., Pittsburgh, PA) while EEG was recorded from the scalp. After viewing a food-related image, each participant was dispensed a chocolate candy, which they had the option to eat or discard, or a bead. Food-related images consisted of sweet or salty contents (for example: cake [sweet], pizza [salty]). One of these two categories of food images (counterbalanced across participants) preceded the delivery of the candy, whereas the other preceded the delivery of the bead. To identify the candy and bead conditions, we decided to use two different categories of food-related images. We counterbalanced the two categories of food images to control for the potential effect of the type of food picture. Each participant was told at the beginning of the EEG session which category of food image would precede the candies and which would precede the beads. The pictures used in this task were selected from the International Affective Picture System (IAPS) [55] and a set of pictures used in our previous studies [18,33]. See Supplementary Table ST1 for a list of the IAPS pictures used in the experiment.
Fig. 1.
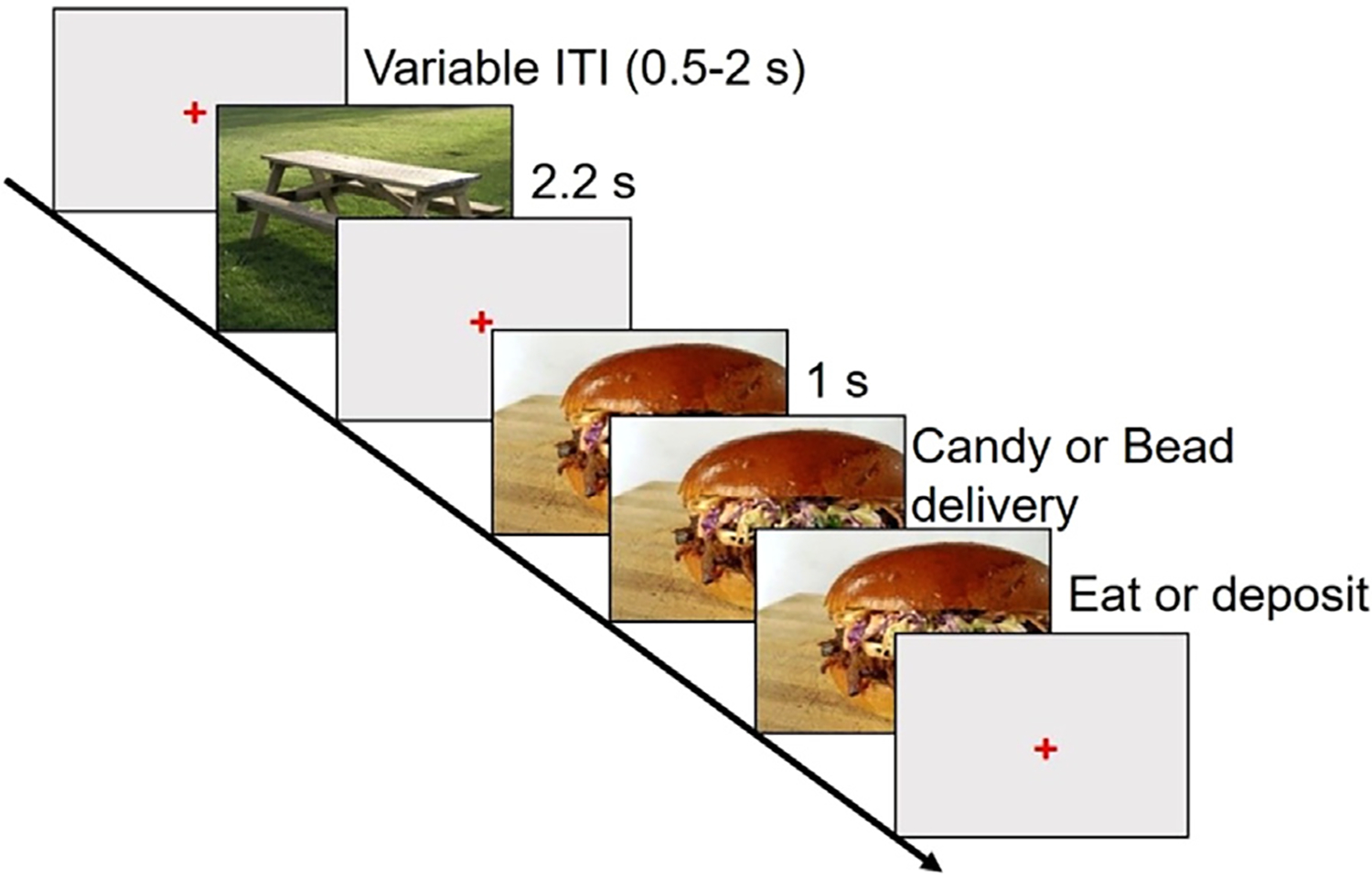
A schematized representation of the timing of events in the cued food delivery task. In this task, participants viewed emotional, neutral, and food-related images and made food-related decisions. After a food-related image was presented on the screen, the participant received either a candy, which they could eat or discard, or a bead. ITI: intertrial interval.
The cued food delivery task consisted of six experimental blocks that lasted about 5 min each. In each block, 55 images were presented pseudorandomly (no more than two images belonging to the same picture category were presented consecutively): 10 neutral (people and objects), 10 pleasant (erotica and romance), 15 unpleasant (mutilations, violence, and pollution), and 20 food-related (salty or sweet) images.
No images were repeated during the task. For the food images, the candy or bead was dispensed 1000 ms after the food cue appeared on the screen through a tube into a receptacle. The participant then could either pick up and eat the candy or discard it in a box. Each food image remained visible until the participant either deposited the candy or bead into the box or pressed a button indicating that they had finished eating the candy. All non-food images were presented for 2.2 s, and a random intertrial interval (ITI) of 3–5 s separated each trial. To familiarize the participants with the task, we ran 11 practice trials, two of which were followed by candy or bead.
2.5. EEG acquisition
We continuously recorded EEG during the task using a 129-channel Geodesic Sensor Net that was amplified with an AC-coupled high-input-impedance (200 MW) amplifier (Geodesic EEG System 200; EGI, Eugene, OR) and referenced to electrode Cz. EEG data were collected at a sampling rate of 250 Hz and filtered online using a 0.1 Hz high-pass and 100 Hz low-pass filter. The scalp impedance was kept under 50 KW as per the manufacturer’s instructions.
2.6. Data reduction
After collecting the EEG, the EEG data were filtered using a 30-Hz low-pass filter and visually inspected to identify broken channels, which were defined as any channels contaminated by artifacts in more than 50% of the recording. Any broken channels were interpolated using spherical splines. Next, the EEG recordings were corrected for blinks and horizontal eye movements using a spatial filtering method implemented in the BESA software program (version 5.1.8.10; MEGIS Software GmbH, Gräfelfing, Germany). The data were then transformed to the average reference and segmented as follows. For the analysis of ERPs, each segment of EEG was time-locked to the onset of each picture in segments that started 1500 ms before the onset of the picture and lasted until 1500 ms afterward. For the time-frequency analyses, each segment of EEG was time-locked to the delivery of a candy or bead in segments that started 1500 ms before the dispensation of the candy or bead and lasted until 1500 ms afterward. For the conditions in which no candy or bead was dispensed, the segments were time-locked to 1000 ms after the presentation of the picture. Thus, time-frequency data for the pleasant, unpleasant, and neutral conditions represent brain activity when the participant does not need to make a response.
The data were baseline-corrected using a 100-ms time bin before the onset of the pictures (ERPs) or the onset of the candy or bead dis- pensation (time-frequency) as the baseline. Artifacts in the −1000- to +1000-ms time window for each segment were then detected based on the following criteria: EEG amplitude above 100 or below −100 mV, an absolute voltage difference between any two points in a segment no greater than 100 mV, maximum voltage step between two contiguous data points of 20 mV, and less than 0.5 mV of variation in activity for more than 100 ms. Channels that were marked bad in more than 40% of the segments were interpolated, and any segment that included more than 12 bad channels after interpolation was discarded. On average, 80% of the segments per condition survived artifact detection. The mean number of retained segments after artifact rejection for each condition is presented in Supplementary Table ST2.
2.7. LPP
We used the amplitude of the LPP as a measure of cues’ motivational salience. To calculate the LPP for each subject and picture category, the EEG responses that were time-locked to the onset of each picture during the 400- to 800-ms time window using a pooled set of centroparietal sensors (EGI HydroCel Geodesic Sensor Net sensors 7, 31, 37, 54, 55, 79, 80, 87, 106, 129; see also Fig. 2 inset) were averaged together. This is the same temporospatial region of interest (ROI) used in our previous studies investigating the LPP [18,19,56].
Fig. 2.
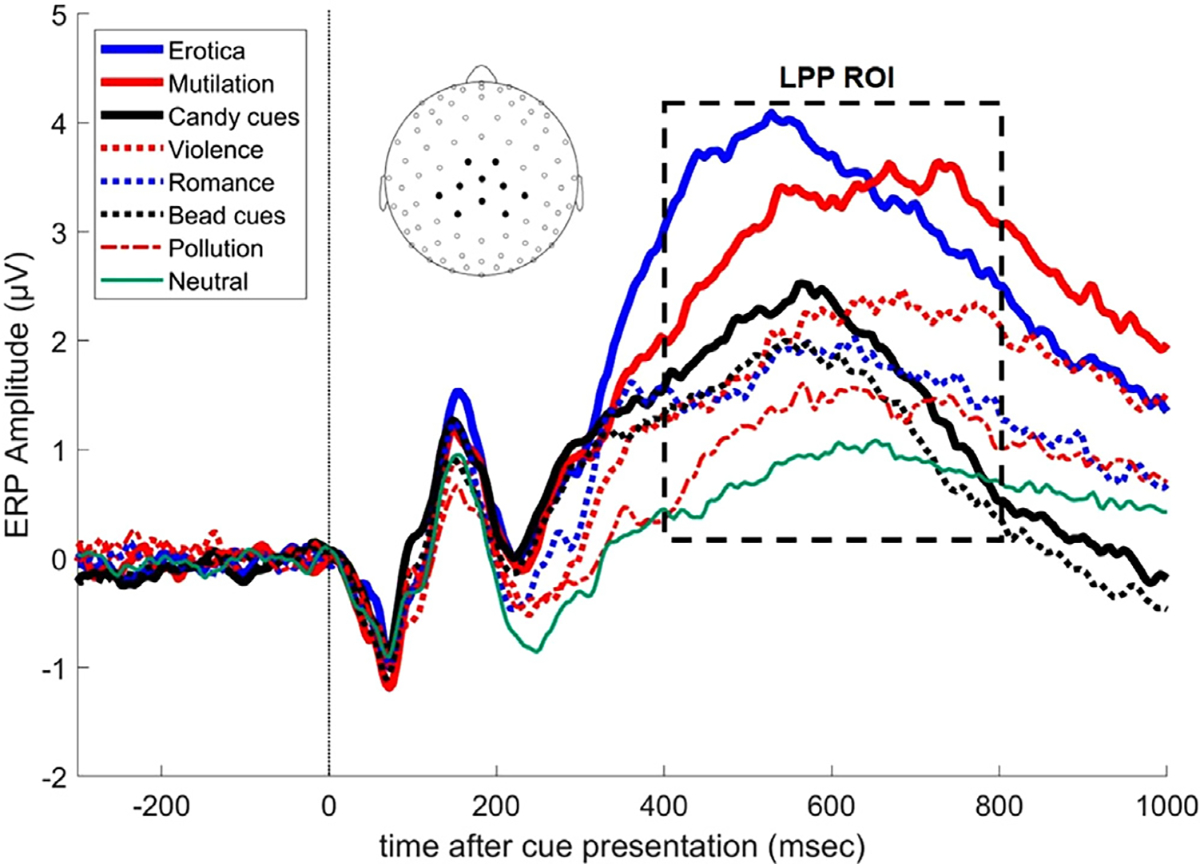
ERPs for centroparietal scalp sites (see inset for EEG electrode locations) showing that, on average, the LPP amplitude was higher for motivationally relevant pictures, such as mutilations or erotic images, than for other types of pictures. The box outlines the temporal ROI used to calculate the LPP for each picture category. Each picture was presented on the screen at time 0.
2.8. Theta power
To calculate theta power, the EEG data time-locked to the delivery of the candy or bead and the corresponding neutral segments were transformed into the time-frequency domain using a continuous wavelet transform. The wavelet transform was based on a complex Morlet wavelet function with a Morlet parameter of 5 using 40 linear frequency steps from 1 to 40 Hz. The data were normalized using Gabor normalization and were baseline-corrected using a reference interval from −875 to −625 ms. To calculate theta power, the 4- to 8-Hz frequency bands were averaged.
Then, we identified the set of EEG sensors to pool together for the analyses using the following procedure. Using theta power as a dependent variable, we performed for each time point and each EEG sensor repeated-measures ANOVA with condition (candy, bead, and neutral) as a factor. We thresholded the F-values resulting from these ANOVAs using Bonferroni correction, and we selected the sensors that showed statistically significant differences during the 0- to 200 ms time region of interest when cognitive control-related effects in theta power are typically greatest [43]. Supplementary Fig. SF3 shows the topographic distribution of the F-values, and the inset of Fig. 4 shows the sensors included in the pool. Fig. 4 shows the time course of theta power in the candy, bead & neutral conditions: on average, power increased when either candies or beads were dispensed to the participant, but not when they passively viewed neutral pictures. To obtain a single theta power value for each participant under the candy, bead, and neutral conditions, we averaged theta power in the 0- to 200-ms time bin from this pooled set of sensors.
Fig. 4.
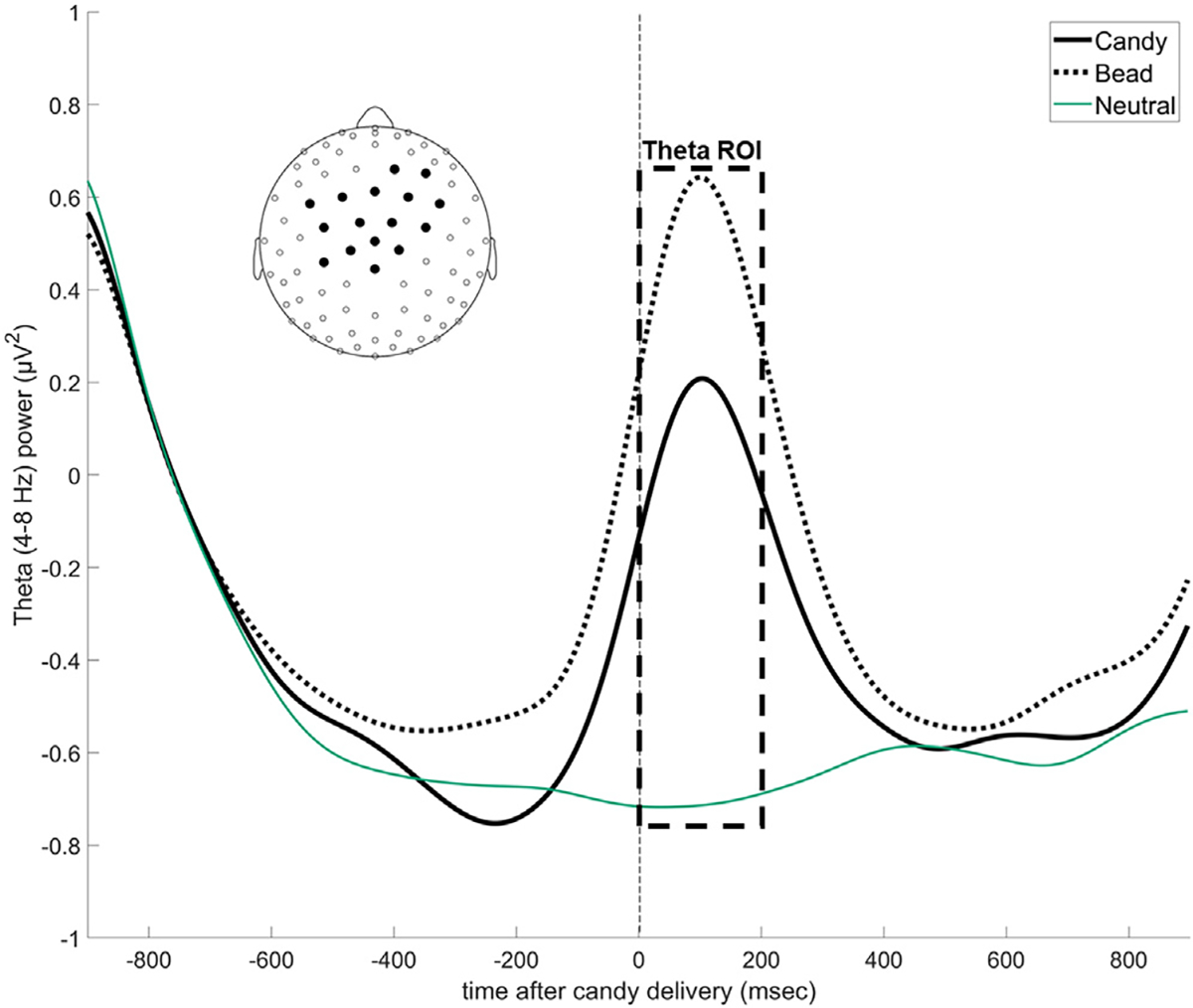
Time series data showing changes in average theta power over mid-frontal scalp sites during the candy, bead & neutral experimental conditions. Theta power over midfrontal scalp sites (see inset for EEG electrode locations) increases during the candy and bead conditions but not when the participant is passively viewing neutral pictures. The box indicates the temporal ROI used to calculate theta power. The candies and beads were delivered at time 0.
2.9. Classification of participants
To classify participants based on their LPP responses to cues, we followed the same procedure as our previous studies [18,33]. First, we z-transformed each participant’s LPP data for each picture subcategory (cues preceding candy, cues preceding beads, erotic, romance, neutral, pollution, violence, and mutilation images), then we applied k-means (k = 2) cluster analysis to the eight z-transformed LPP values. The number of clusters (k = 2) was decided a priori based on previous findings [33,56].
To classify the participants based on their theta power amplitudes, we followed a similar strategy Specifically, we computed theta power during a 0- to 200-ms time window across a pooled set of mid-frontal sensors for the candy, bead, and neutral conditions. We z-transformed these values and then applied a k-means (k = 2) clustering algorithm, with the a priori hypothesis that two distinct patterns of theta activity would be observed, much like our previous findings using the LPP.
2.10. Eating behavior
Because the number of candies the participants ate during the experiment is a count variable, we tested differences in eating behavior between participant groups using Poisson regression analysis. First, we compared the number of candies eaten during the experiment between the two LPP-derived groups. Second, we conducted another Poisson analysis to compare the number of candies eaten between the two theta power-derived groups. Third, we compared the number of candies eaten by the four groups formed by crossing the LPP and theta power-based groups using Poisson regression.
2.11. Demographics and questionnaires
To identify whether any demographic or psychological factors had confounding effects on the trends in eating behavior we observed in the participant groups, we conducted Poisson regression modeling the effect of group assignment (LPP and theta power) on eating behavior. Demographic (age, gender, race, ethnicity), body mass index (BMI), and questionnaire data (SLIM, PFS, FCQ, WREQ, BIS, and PANAS) were included in the model as covariates.
3. Results
3.1. ERPs
Fig. 2 shows the grand averaged ERPs for each picture category. As expected, the amplitude of the LPP increased as a function of motivational salience irrespective of hedonic content. We formally tested this effect using LPP amplitude as a dependent variable in a repeated-measures analysis of variance (ANOVA) with the picture category as an eight-level factor (candy cues, bead cues, erotica, romance, neutral, pollution, violence, and mutilations; F[7, 399] = 22.1, p < 0.001). We also tested the quadratic trend of increasing LPP as a function of motivational salience under both pleasant and unpleasant conditions (F[5, 290] = 46.3, p < 0.001). Furthermore, we found that on average, food images preceding the dispensation of the candy elicited larger LPPs than did food images preceding the dispensation of the bead (F[1, 58] = 5.02, p = 0.029).
We tested whether the type of food picture (salty or sweet) influenced the LPP responses to the candy and bead conditions. When the candy and bead conditions are collapsed together, we found that the amplitude of the LPP is larger on average in response to sweet cues than in response to salty cues. However, this difference was not statistically significant (F[1, 58] = 1.98, p = 0.165).
We then separated the candy and bead responses, finding that, for the candy condition, pictures of sweet foods elicited significantly larger LPPs than pictures of salty foods (F[1, 57] = 5.40, p = 0.024). For the bead condition, the effect of picture type was non-significant (F[1, 57] = 0.92, p = 0.34). The average LPP responses for candy and bead conditions by picture type are shown in Supplementary Figure SF1.
3.2. Classification of participants: LPP
Fig. 3A shows the mean LPP responses for each picture category within the two groups identified by the cluster analysis. Both groups exhibited the canonical pattern of progressively larger LPP responses for both pleasant and unpleasant images as a function of their motivational salience (C>P group: F = 87.5, p < 0.001; P>C group: F = 77, p < 0.001). However, one group had significantly larger LPP responses to pleasant images (erotic and romantic picture subcategories averaged together) than to cues preceding food rewards (P>C group; F = 6.51, p = 0.013), whereas the other had significantly larger LPP responses to cues preceding food rewards than to pleasant images (C>P group; F = 59, p < 0.001). See Supplementary Figure SF2 for the averaged LPP responses to the parent picture categories (pleasant, unpleasant, and neutral) for each group.
Fig. 3.
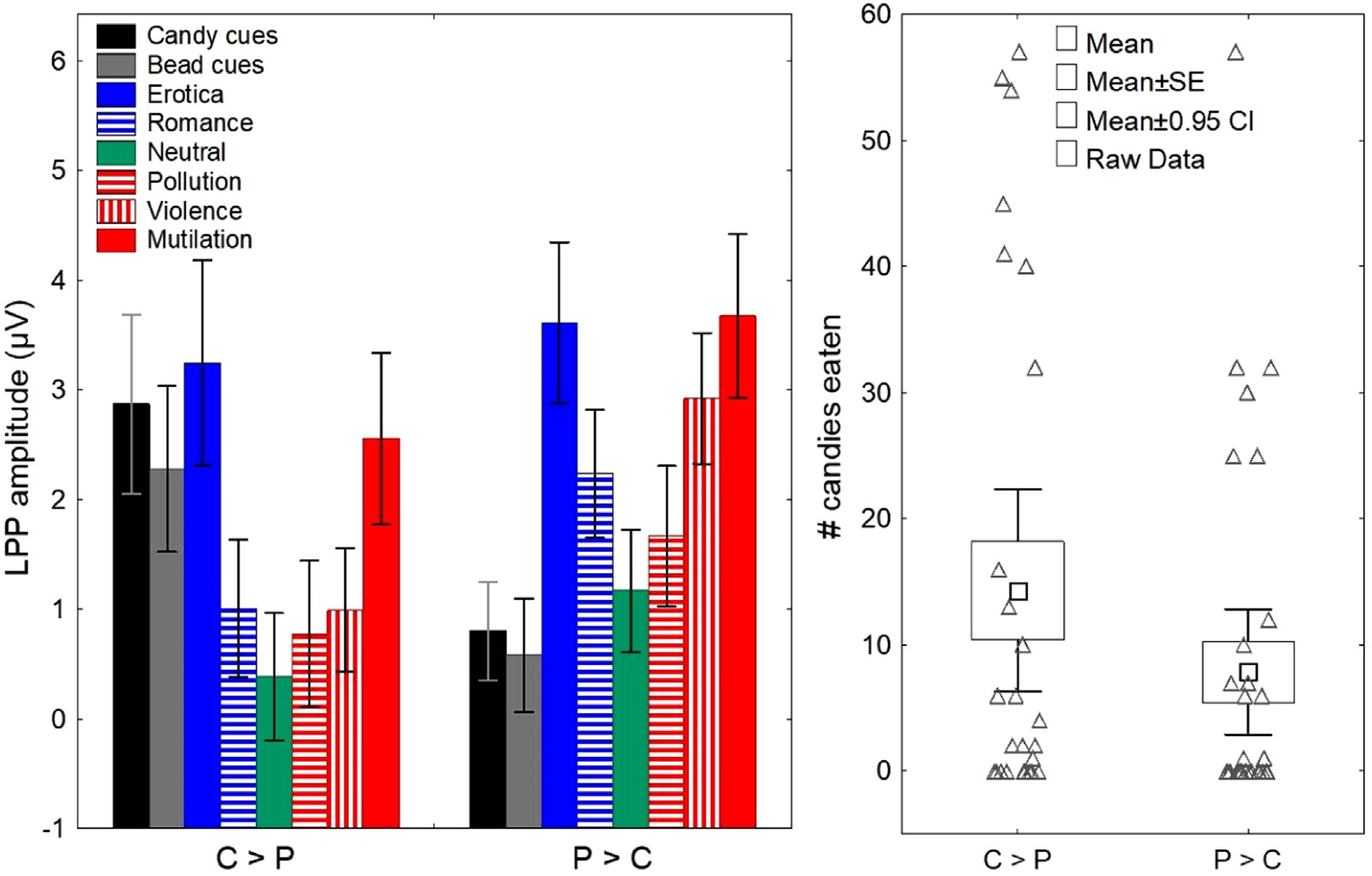
We replicated the finding that individual differences in LPP responses to food cues & non-food-related pleasant images are predictive of cue-induced eating. (A) K-means clustering of the LPP responses identified two groups: one with higher LPP amplitudes for food cues than for pleasant images (C>P group) and one with higher LPP amplitudes for pleasant images than for food cues (P>C group). Both groups exhibited the canonical pattern of progressively larger LPP responses for both pleasant and unpleasant images as a function of their motivational salience. Error bars: 95% CI. (B) The C>P group ate significantly more candies during the cued food delivery task than did the P>C group. SE: standard error. Error bars: 95% CIs.
After determining the group assignment for each participant, we compared the number of candies eaten by the C>P and P>C groups (Fig. 3B and Supplementary Table ST3). The results of the Poisson regression analysis showed that participants in the C>P group consumed significantly more candies during the experiment than those in the P>C group (Wald X2[1] = 43.1, p < 0.001). Table 1 shows that demographic, biometric, and self-reported questionnaire data were comparable across the C>P and P>C groups.
We then tested for differences in eating behavior between the C>P and P>C groups among those participants who saw sweet food pictures preceding candy versus those who saw salty food pictures preceding candy. Among the participants who saw sweet food pictures before they were dispensed candy, individuals in the C>P group ate significantly more than those in the P>C group (Wald X2[1] = 39.25, p < 0.001). Furthermore, among the participants who saw salty food pictures before they were dispensed candy, individuals in the C>P also ate significantly more than those in the P>C group (Wald X2[1] = 6.72, p = 0.0095). Thus, both cue types elicited the same dynamic in eating behavior between C>P and P>C groups.
3.3. Time-frequency analyses
We conducted an ANOVA to determine whether theta power under the candy, bead, and neutral conditions differed between C>P and P>C groups and found no significant interaction effect of group (C>P and P>C) and condition (candy, bead, and neutral) (F[2, 114] = 0.667, p = 0.515). The mean values for each condition within the C>P and P>C groups are shown in Supplementary Fig. SF4. This result indicates that attributing high incentive salience to food-related cues (as measured by the LPP) is not systematically associated with reduced cognitive control in the presence of the cues. Hence, we used individual differences in theta power responses to the delivery of candies and beads to classify participants.
3.4. Classification of participants: theta power
When we tested for differences in theta power under the candy and bead conditions for the entire participant sample, we found that, when averaging all the participants together, theta power is significantly higher for the bead condition than the candy condition (F[1, 58] = 12.62, p = 0.001). However, Fig. 5A shows that the two groups identified by the cluster analysis had almost identical values of theta power for the neutral condition (when no decisions were made and participants passively viewed the images) but differed in the extent to which theta power increased when candies or beads were delivered and participants decided the fate of these items. In one group (θCA>θBE) theta power increased more for the candy condition than for the bead condition (F = 9.62, p = 0.003), while in the other group (θBE>θCA) theta power increased more for the bead condition than for the candy condition (F = 77.43, p < 0.001).
Fig. 5.
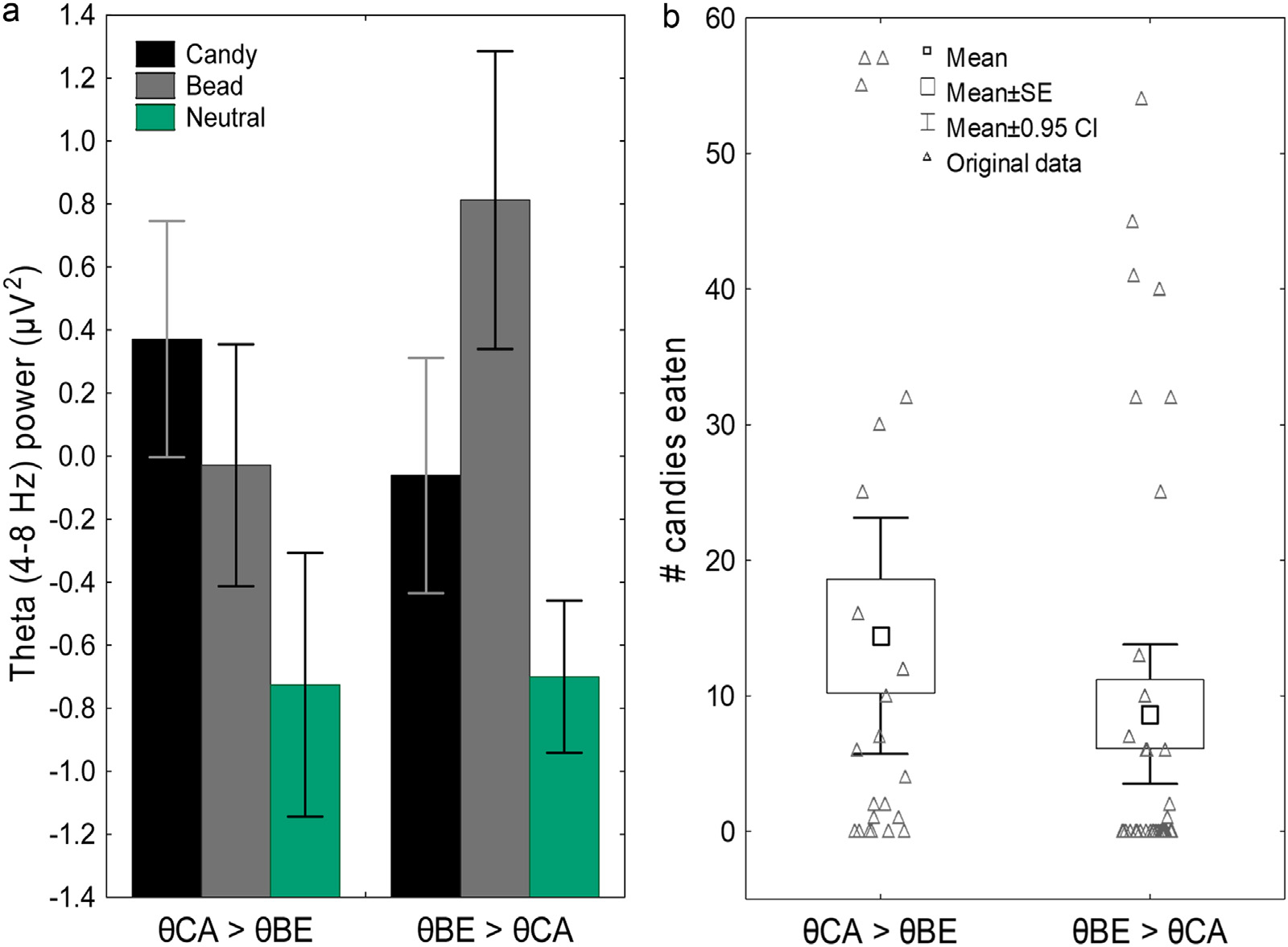
We found that individual differences in theta power during food-related decision-making were predictive of cue-induced eating. (A) K-means clustering of theta power data identified two groups: one with higher theta power for the candy condition than for the bead condition (θCA>θBE group) and one with higher theta power for the bead condition than for the candy condition (θBE>θCA group). Error bars: 95% CIs. (B) The θCA>θBE group ate significantly more candies during the cued food delivery task than did the θBE>θCA group. SE: standard error. Error bars: 95% CI.
We then compared the number of candies eaten by the two groups during the experiment (See Fig. 5B and Supplementary Table ST3). The results of the Poisson regression analysis indicated that the participants assigned to the θCA>θBE group ate significantly more candies than those assigned to the θBE>θCA group (Wald X2[1] = 41.5, p < 0.001). Demographic, biometric, and self-reported questionnaire responses did not differ between the θCA>θBE and θBE>θCA groups and are reported in Table 1.
3.5. Classification of participants: LPP and theta power
To explore how individual differences in both the attribution of motivational salience to food cues and the engagement of cognitive control contribute to cue-induced eating, we created four participant groups by combining the outcomes of the LPP and theta power classification procedures. We labeled the four groups: P>C & θBE>θCA, P>C & θCA>θBE, C>P & θBE>θCA, and C>P & θCA>θBE. Demographic, biometric, and self-reported questionnaire data for the four groups are reported in Table 2.
The results of the Poisson regression analysis indicated a significant effect of group assignment on the number of candies eaten during the experiment (Wald X2[3] = 106.2, p < 0.001). Individuals in the P>C & θBE>θCA group ate the lowest number of candies, while those in the other three groups consumed a similar number of candies on average (Wald X2[2] = 0.825, p = 0.662; See Fig. 6 and Supplementary Table ST3).
Fig. 6.
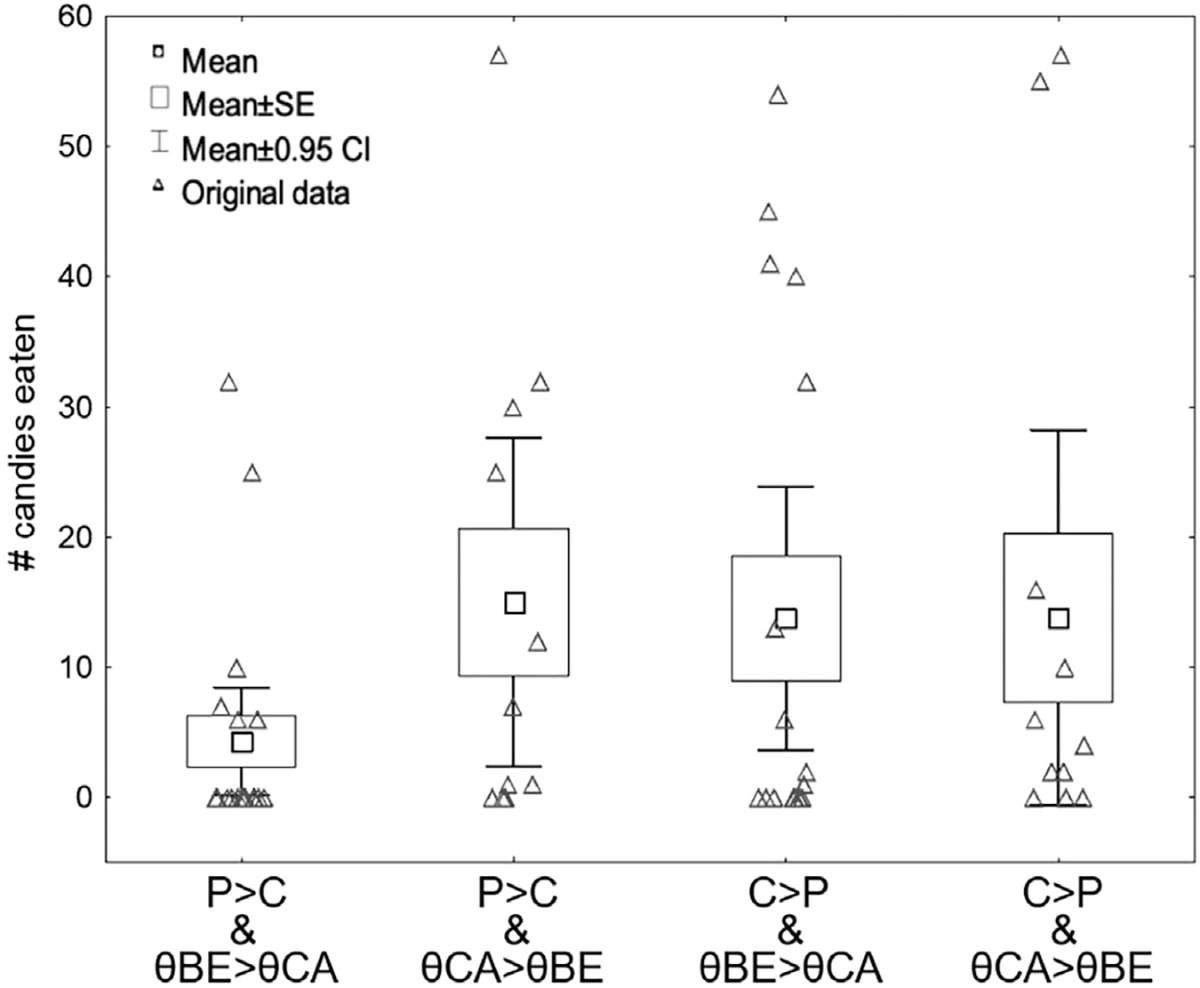
Number of candies eaten per group by crossed LPP and theta power groups. After crossing the participant groups according to the LPP and theta-based cluster analyses, we found that individuals with neither LPP nor theta-based “risk factors” (P>C and θBE>θCA group) ate the least of all four groups. The three remaining groups exhibited similar levels of eating behavior on average. SE: standard error. Error bars: 95% CIs.
Finally, we examined whether cue type (sweet vs salty food images) had an effect on P>C vs C>P group assignment or subsequent eating behavior. We conducted a χ2 test to determine if there was a difference in the number of individuals categorized as C>P or P>C between those who saw sweet vs salty food images preceding candy rewards. We found no significant differences in P>C vs C>P group assignment between those who saw sweet vs salty cues preceding candy rewards (χ2 = 2.08, p = 0.15). We also tested for differences in eating behavior between those who saw sweet vs salty food images and found no significant effect (Wald X2[1] = 1.84, p = 0.18; see Supplementary Figure SF5).
3.6. Effects of covariates on candy consumption
To account for the potential influence of confounding variables on the association between the psychophysiologically-derived groups and candy consumption, we progressively added covariates to the Poisson regression model described above. The covariates that we incorporated into the model included demographic information (age, gender, race, ethnicity), BMI, and responses to questionnaires assessing hunger and satiety at the beginning of the experiment [47], eating behaviors [49,50], sensitivity to reward and punishment [57], mood [52], and impulsivity [51]. The significant main effect of group on eating behavior remained consistent in terms of both sign and magnitude across all iterations of the model. In the final model, which controlled for all the aforementioned covariates, the difference in the number of candies eaten relative to the P>C&θ BE>θCA group was significant for every group except the C>P & θCA>θBE group, where it was marginally non-significant (p = 0.056). The detailed output of this final model is presented in Table 3.
Table 3.
Poisson regression modeling the effect of crossed group assignment on eating behavior after controlling for demographic, biometric and self-report data.
| IRR | SE | z | P > |z| | 95% CI | |
|---|---|---|---|---|---|
|
| |||||
| Crossed LPP & Theta Group | |||||
| P>C & θCA>θBE | 2.17 | 0.35 | 4.82 | 0.00 | 1.58 2.98 |
| C>P & θBE>θCA | 3.59 | 0.57 | 8.09 | 0.00 | 2.64 4.90 |
| C>P & θCA>θBE | 1.40 | 0.24 | 1.91 | 0.056 | 0.99 1.96 |
| Age | 1.03 | 0.005 | 6.79 | 0.00 | 1.02 1.04 |
| Gender | 3.36 | 0.46 | 8.91 | 0.00 | 2.57 4.39 |
| Race Black/African American |
0.51 | 0.10 | −3.56 | 0.00 | 0.35 0.74 |
| I prefer not to say | 3.43e-06 | 0.001 | −0.03 | 0.97 | 0.00 0.00 |
| More than one race | 0.27 | −1.26 | 0.28 | 0.21 | 0.03 2.09 |
| White | 0.95 | 0.17 | −0.26 | 0.80 | 0.67 1.36 |
| BMI | 1.00 | 0.01 | −1.06 | 0.29 | 0.98 1.01 |
| SLIM | 1.00 | 0.00 | −3.60 | 0.00 | 0.99 1.00 |
| PFS | 0.97 | 0.01 | −5.49 | 0.00 | 0.96 0.98 |
| FCQ | 0.97 | 0.00 | −10.54 | 0.00 | 0.96 0.97 |
| WREQ-S | 2.74 | 0.25 | 11.28 | 0.00 | 2.30 3.27 |
| BIS AT | 1.08 | 0.02 | 4.04 | 0.00 | 1.04 1.12 |
| BIS MO | 1.08 | 0.02 | 4.38 | 0.00 | 1.04 1.11 |
| BIS NO | 0.74 | 0.02 | −11.72 | 0.00 | 0.71 0.78 |
| PANAS − | 1.00 | 0.01 | 0.44 | 0.66 | 0.99 1.02 |
| PANAS + | 0.99 | 0.01 | −1.99 | 0.047 | 0.98 1.00 |
| Intercept | 12.34 | 7.04 | 4.41 | 0.00 | 4.04 37.73 |
IRR: Incidence rate ratio, SE: standard error, CI: confidence interval. WREQ-S: Weight-related Eating Questionnaire, susceptibility to external cues subscale; BIS-AT: Barratt Impulsivity Scale, attention subscale; BIS-MO: Barratt Impulsiveness Scale, motor impulsiveness subscale; BIS NO: Barratt Impulsiveness Scale, non-planning subscale; PANAS−: Positive and Negative Affect Schedule, negative affect subscale; PANAS + : Positive and Negative Affect Schedule, positive affect subscale.
4. Discussion
This study aimed at investigating how individual differences in affective and cognitive responses to cues predicting food rewards contribute to the regulation of cue-induced eating in humans. The rationale for this work was informed by results from animal models demonstrating that individual differences in the tendency to attribute motivational salience to food-related cues and top-down attentional control mechanisms influence vulnerability to cue-induced compulsive reward-seeking behaviors. We found that both higher LPP responses to food-related cues (a measure of motivational salience) and higher power in the EEG theta band (a measure of cognitive control) were independently associated with higher food consumption during the experiment. The results from this study provide insights into the psychophysiological mechanisms underlying maladaptive eating behaviors and could potentially inform the development of new target biomarkers for the treatment of such behaviors.
The amplitude of the LPP is a reliable electrophysiological measure of the incentive salience that individuals attribute to stimuli in the environment [24,25]. By applying cluster analysis to the LPP responses evoked by food-related and non-food-related motivationally salient images, we identified two reactivity profiles associated with vulnerability to cue-induced eating: individuals with larger LPP responses to food-related cues than to pleasant images (C>P group) ate significantly more than did individuals with larger LPP responses to pleasant stimuli than to food-related cues (P>C group). These results replicate those from previous studies [18,33] and support the hypothesis that individual differences in the tendency to attribute motivational salience to food-related cues than to other pleasant stimuli underlie vulnerability to cue-induced eating [12,19,58,59].
Furthermore, we found that midfrontal theta power increased after the delivery of candies and beads and that individual differences in mid-frontal theta power were associated with vulnerability to cue-induced eating. Specifically, individuals with higher phasic theta power following the delivery of candies than of beads (θCA>θBE) ate more during the cued food delivery task than those with the opposite theta response pattern (θBE>θCA). Phasic changes in theta power over midfrontal scalp sites have been proposed as an index of the engagement of cognitive control mechanisms [43] because midfrontal theta tends to increase when an individual is executing a task that requires increased attentional demands, such as inhibiting prepotent responses [40, 41] and performing cognitively demanding tasks [42]. In light of these findings, the results of the present study suggest that some individuals struggle with food-related decision-making and that these individuals are more likely to engage in cue-induced eating when a palatable food option is available [11,60].
Finally, when we crossed the group assignments from the LPP and theta-based classification procedures, we identified four groups with roughly the same number of subjects in them. While individuals with neither the LPP nor the theta risk factor (i.e., the P>C & θBE>θCA group) ate the least number of candies, the presence of either risk factor significantly increased cue-induced eating. These results suggest that the LPP-based and q-based reactivity profiles reflect affective and cognitive mechanisms that independently modulate an individual’s propensity to cue-induced eating.
Our observation that the C>P and P>C groups had similar theta power dynamics during food-related decision-making is inconsistent with results from animal models: rats that attribute high motivational salience to food-related cues are also more impulsive and less able to implement top-down attentional control in the presence of cues compared to rats that do not attribute high motivational silence to food-related cues [12,61–63]. This difference might be explained by the fact that theta power dynamics recorded during the cued food availability task may not reflect the same top-down attentional control and impulsivity mechanisms that are involved in animal models. Also, our self-reported data did not show significant differences in impulsivity scores between groups, which may explain the divergent findings of the present study and those in the animal literature. Inherent differences between humans and animal models [58], especially when executive control and higher cognitive functions are central for the execution of a task [11], make direct comparisons of human and animal results difficult. Further studies leveraging the paradigm that we used here should facilitate the bidirectional translation and improvement of both animal models and human research investigating cue-induced behavior [31,64]. while the validity of the LPP as a predictor of cue-induced behaviors has been well replicated [33,56,65,66] and is consistent with theoretical models concerning the motivational salience of cues [12,44], the predictive validity of the theta-based correlate as described in the present study is novel and should be considered preliminary until replicated. Although in previous studies researchers showed that frontal theta power increases during the execution of cognitively demanding tasks and theta power has been proposed as an index of the engagement of cognitive control [43], here we did not explicitly manipulate cognitive load during food-related decision-making. Hence, inferring from our results that the observed dynamics in theta power reflect the differential engagement of cognitive control during food-related decision-making remains speculative. In fact, in addition to top-down control, the dynamics in theta power that we observed here might also partially reflect the salience of the cues. It has been shown that power in the theta frequency band is lower in response to salient images compared to neutral images [67]. Thus, theta power may be lower in the candy condition than in the bead condition (as shown in Fig. 4) because cues predicting food delivery hold higher motivational salience.
Despite these limitations, our findings are consistent with neurobiological models suggesting that both high reactivity to food-related cues and impaired cognitive control can contribute to excessive eating [8]. By identifying individual risk factors that increase vulnerability to cue-induced eating, our approach may inform the development of personalized treatments for individuals who struggle with overeating. Such interventions may include treatments that focus on reducing the salience of food-related stimuli or strategies to enhance cognitive control in the presence of food cues. For example, repetitive transcranial magnetic stimulation (rTMS) [68] can non-invasively upregulate brain activity in cognitive control networks [69] or downregulate brain activity in reward networks [70]. Thus, a patient identified to have high affective vulnerability may be selected to receive inhibitory rTMS of the ventromedial prefrontal cortex, which is commonly implicated in reward processing [71], while a patient with cognitive control vulnerability may be more effectively treated with excitatory rTMS of the dorsolateral prefrontal cortex, which is commonly implicated in executive control [72].
In conclusion, our results demonstrated that individual differences in both the amplitude of the LPP and theta power are predictive of cue-induced eating behavior, suggesting that both affective and cognitive mechanisms are implicated in the regulation of cue-induced eating. By simultaneously measuring both the amplitude of the LPP and theta power while participants were in the presence of food-related cues and actual food rewards, we clarified the mechanisms underlying cue-induced eating behaviors. Continuing this line of investigation may inform clinicians of mechanisms underlying maladaptive eating and may foster the development of personalized clinical interventions for excessive eating.
Supplementary Material
Acknowledgment
We thank Drs. Scott Lane and Andreas Keil for advising us in the analysis of these data and MD Anderson Editing Services, Research Medical Library for assistance in editing the manuscript.
Funding
This work was supported by the National Institute on Drug Abuse (1F31DA054702–01 [to KDG] and R01DA032581 [to FV]) and the NIH/NCI under award number P30CA016672. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- ANOVA
analysis of variance
- BIS
Barratt Impulsiveness Scale
- C>P
LPP responses that are larger to food cues than to pleasant images
- EEG
electroencephalogram
- ERP
event-related potential
- LPP
late positive potential
- P>C
LPP responses that are larger to pleasant images than to food cues
- PANAS
Positive and Negative Affect Scale
- ROI
region of interest
- SLIM
Satiety Labeled Intensity Magnitude
- θBE>θCA
theta power that is higher under the bead condition than under the candy condition
- θCA>θBE
theta power that is higher under the candy condition than under the bead condition
- WREQ
weight-related eating questionnaire
Footnotes
Declaration of Competing Interest
The authors declare no conflicts.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.addicn.2023.100106.
Data availability
Data will be made available on request.
References
- [1].Prospective Studies Collaboration, Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies, Lancet 373 (2009) 14, doi: 10.1016/SO140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jeffery RW, Drenowski A, Epstein LH, Stunkard AJ, Wilson TG, Wing RR, Long-term maintenance of weight loss: current status, Health Psychol. 19 (2000) 5–16, doi: 10.1037//0278-6133.19.1. [DOI] [PubMed] [Google Scholar]
- [3].Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE, Randomized clinical trials of weight loss maintenance: a review, J. Cardiovasc. Nurs. 24 (2009) 58–80, doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Davis AA, Edge PJ, Gold MS, New directions in the pharmacological treatment of food addiction, overeating, and obesity, Behav. Addict (2014) 185–213 Elsevier, doi: 10.1016/B978-0-12-407724-9.00008-2. [DOI] [Google Scholar]
- [5].de Lauzon-Guillain B, Clifton EA, Day FR, Clément K, Brage S, Forouhi NG, Griffin SJ, Koudou YA, Pelloux V, Wareham NJ, Charles MA, Heude B, Ong KK, Mediation and modification of genetic susceptibility to obesity by eating behaviors, Am. J. Clin. Nutr. 106 (2017) 996–1004, doi: 10.3945/ajcn.117.157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Appelhans BM, Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity, Obesity 17 (2009) 640–647, doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- [7].van den Bos R, de Ridder D, Evolved to satisfy our immediate needs: self-control and the rewarding properties of food, Appetite 47 (2006) 24–29, doi: 10.1016/j.appet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- [8].Stice E, Yokum S, Neural vulnerability factors that increase risk for future weight gain, Psychol. Bull. 142 (2016) 447–471, doi: 10.1037/bul0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pitchers KK, Kane LF, Kim Y, Robinson TE, Sarter M, Hot’ vs. ‘cold’ behavioural-cognitive styles: motivational-dopaminergic vs. cognitive-cholinergic processing of a Pavlovian cocaine cue in sign- and goal-tracking rats, Eur. J. Neurosci. 46 (2017) 2768–2781, doi: 10.1111/ejn.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pitchers KK, Phillips KB, Jones JL, Robinson TE, Sarter M, Diverse roads to relapse: a discriminative cue signaling cocaine availability is more effective in renewing cocaine seeking in goal trackers than sign trackers and depends on basal forebrain cholinergic activity, J. Neurosci. 37 (2017) 7198–7208, doi: 10.1523/JNEUROSCI.0990-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hall PA, Executive-control processes in high-calorie food consumption, Curr. Dir. Psychol. Sci. 25 (2016) 91–98, doi: 10.1177/0963721415625049. [DOI] [Google Scholar]
- [12].Sarter M, Phillips KB, The neuroscience of cognitive-motivational styles: sign- and goal-trackers as animal models, Behav. Neurosci 132 (2018) 1–12, doi: 10.1037/bne0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fitzpatrick CJ, Morrow JD, Pavlovian conditioned approach training in rats, J. Vis. Exp 53580 (2016), doi: 10.3791/53580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morrison SE, Bamkole MA, Nicola SM, Sign tracking, but not goal tracking, is resistant to outcome devaluation, Front. Neurosci. 9 (2015), doi: 10.3389/fnins.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fitzpatrick CJ, Geary T, Creeden JF, Morrow JD, Sign-tracking behavior is difficult to extinguish and resistant to multiple cognitive enhancers, Neurobiol. Learn. Mem. 163 (2019) 107045, doi: 10.1016/j.nlm.2019.107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garofalo S, di Pellegrino G, Individual differences in the influence of task-irrelevant Pavlovian cues on human behavior, Front. Behav. Neurosci 9 (2015), doi: 10.3389/fnbeh.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schad DJ, Rapp MA, Garbusow M, Nebe S, Sebold M, Obst E, Sommer C, Deserno L, Rabovsky M, Friedel E, Romanczuk-Seiferth N, Wittchen H-U, Zimmermann US, Walter H, Sterzer P, Smolka MN, Schlagenhauf F, Heinz A, Dayan P, Huys QJM, Dissociating neural learning signals in human sign- and goal-trackers, Nat. Hum. Behav (2019), doi: 10.1038/s41562-019-0765-5. [DOI] [PubMed] [Google Scholar]
- [18].Kypriotakis Versace G, Basen-Engquist K, Schembre SM, Heterogeneity in brain reactivity to pleasant and food cues: evidence of sign-tracking in humans, Soc. Cogn. Affect. Neurosci 11 (2016) 604–611, doi: 10.1093/scan/nsv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Versace F, Engelmann JM, Deweese MM, Robinson JD, Green CE, Lam CY, Minnix JA, Karam-Hage MA, Wetter DW, Schembre SM, Cinciripini PM, Beyond cue reactivity: non-drug-related motivationally relevant stimuli are necessary to understand reactivity to drug-related cues, Nicotine Tob. Res. 19 (2017) 663–669, doi: 10.1093/ntr/ntx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deweese MM, Claiborne KN, Ng J, Dirba DD, Stewart HL, Schembre SM, Versace F, Dispensing apparatus for use in a cued food delivery task, MethodsX 2 (2015) 446–457, doi: 10.1016/j.mex.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ, Brain potentials in affective picture processing: covariation with autonomic arousal and affective report, Biol. Psychol 52 (2000) 95–111, doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- [22].Minnix JA, Versace F, Robinson JD, Lam CY, Engelmann JM, Cui Y, Brown VL, Cinciripini PM, The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: A content comparison, Int. J. Psychophysiol 89 (2013) 18–25, doi: 10.1016/j.ijpsycho.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Weinberg A, Hajcak G, Beyond good and evil: the time-course of neural activity elicited by specific picture content, Emotion 10 (2010) 767–782, doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- [24].Lang PJ, Bradley MM, Emotion and the motivational brain, Biol. Psychol 84 (2010) 437–450, doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Webber HE, de Dios C, Kessler DA, Schmitz JM, Lane SD, Suchting R, Late positive potential as a candidate biomarker of motivational relevance in substance use: Evidence from a meta-analysis, Neurosci. Biobehav. Rev 141 (2022) 104835, doi: 10.1016/j.neubiorev.2022.104835. [DOI] [PubMed] [Google Scholar]
- [26].Cofresí RU, Piasecki TM, Hajcak G, Bartholow BD, Internal consistency and test–retest reliability of the P3 event-related potential (ERP) elicited by alcoholic and non-alcoholic beverage pictures, Psychophysiology 59 (2022), doi: 10.1111/psyp.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huffmeijer R, Bakermans-Kranenburg MJ, Alink LRA, van IJzendoorn MH, Reliability of event-related potentials: The influence of number of trials and electrodes, Physiol. Behav. 130 (2014) 13–22, doi: 10.1016/j.physbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- [28].Moran TP, Jendrusina AA, Moser JS, The psychometric properties of the late positive potential during emotion processing and regulation, Brain Res. 1516 (2013) 66–75, doi: 10.1016/j.brainres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- [29].Codispoti M, Mazzetti M, Bradley M, Unmasking emotion: exposure duration and emotional engagement, Psychophysiology 46 (2009), doi: 10.1111/j.1469-8986.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- [30].Codispoti M, De Cesarei A, Ferrari V, The influence of color on emotional perception of natural scenes, Psychophysiology 49 (2012), doi: 10.1111/j.1469-8986.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- [31].Versace F, Robinson JD, Cinciripini PM, Toward neuromarkers for tailored smoking cessation treatments, Addict. Neurosci. 6 (2023) 100075, doi: 10.1016/j.addicn.2023.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gore PA, Tinsley HEA, Brown SD, 11 - Cluster Analysis, in: Handbook of Applied Multivariate Statistics and Mathematical Modeling, Academic Press, San Diego, 2000, pp. 297–321, doi: 10.1016/B978-012691360-6/50012-4. [DOI] [Google Scholar]
- [33].Versace F, Frank DW, Stevens EM, Deweese MM, Guindani M, Schem-bre SM, The reality of “food porn “: Larger brain responses to food-related cues than to erotic images predict cue-induced eating, Psychophysiology (2018) e13309, doi: 10.1111/psyp.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sandre A, Weinberg A, Neither wrong nor right: Theta and delta power increase during performance monitoring under conditions of uncertainty, Int. J. Psychophysiol 146 (2019) 225–239, doi: 10.1016/j.ijpsycho.2019.09.015. [DOI] [PubMed] [Google Scholar]
- [35].Vidal F, Hasbroucq T, Grapperon J, Bonnet M, Is the ‘error negativity’ specific to errors? Biol. Psychol. 51 (2000) 109–128, doi: 10.1016/S0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- [36].Sehrig S, Weiss A, Miller GA, Rockstroh B, Decision- and feedback-related brain potentials reveal risk processing mechanisms in patients with alcohol use disorder, Psychophysiology (2019), doi: 10.1111/psyp.13450. [DOI] [PubMed] [Google Scholar]
- [37].Wang CH, Yang CT, D Moreau NG Muggleton, Motor expertise modulates neural oscillations and temporal dynamics of cognitive control, NeuroImage 158 (2017) 260–270, doi: 10.1016/j.neuroimage.2017.07.009. [DOI] [PubMed] [Google Scholar]
- [38].Carbine KA, Rodeback R, Modersitzki E, Miner M, LeCheminant JD, Larson MJ, The utility of event-related potentials (ERPs) in understanding food-related cognition: A systematic review and recommendations, Appetite 128 (2018) 58–78, doi: 10.1016/j.appet.2018.05.135. [DOI] [PubMed] [Google Scholar]
- [39].Zambrano-Vazquez Cavanagh L, Allen JJB, Theta lingua franca: a common midfrontal substrate for action monitoring processes: omnipresent theta, Psychophysiology 49 (2012) 220–238, doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Haciahmet CC, Frings C, Pastötter B, Target amplification and distractor inhibition: theta oscillatory dynamics of selective attention in a flanker task, Cogn. Affect. Behav. Neurosci. 21 (2021) 355–371, doi: 10.3758/s13415-021-00876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nigbur R, Ivanova G, Stürmer B, Theta power as a marker for cognitive interference, Clin. Neurophysiol. 122 (2011) 2185–2194, doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- [42].Wang L, Chang W, Krebs RM, Boehler CN, Theeuwes J, Zhou X, Neural dynamics of reward-induced response activation and inhibition, Cereb. Cortex (2018) 3961–3976, doi: 10.1093/cercor/bhy275. [DOI] [PubMed] [Google Scholar]
- [43].Cavanagh JF, Frank MJ, Frontal theta as a mechanism for cognitive control, Trends Cogn. Sci. 18 (2014) 414–421, doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pitchers KK, Sarter M, Robinson TE, The hot ‘n’ cold of cue-induced drug relapse, Learn. Mem. 25 (2018) 474–480, doi: 10.1101/lm.046995.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Robinson TE, Berridge KC, Addiction, Annu. Rev. Psychol. 54 (2003) 25–53, doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- [46].Tunstall BJ, Kearns DN, Sign-tracking predicts increased choice of cocaine over food in rats, Behav. Brain Res. 281 (2015) 222–228, doi: 10.1016/j.bbr.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cardello AV, Schutz HG, Lesher LL, Merrill E, Development and testing of a labeled magnitude scale of perceived satiety, Appetite 44 (2005) 1–13, doi: 10.1016/j.appet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [48].Schembre SM, Geller KS, Psychometric properties and construct validity of the weight-related eating questionnaire in a diverse population, Obesity 19 (2011) 2336–2344, doi: 10.1038/oby.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert M, Halford J, The Power of food scale. a new measure of the psychological influence of the food environment, Appetite 53 (2009) 114–118, doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- [50].Nijs IMT, Franken IHA, Muris P, The modified trait and state food-cravings questionnaires: development and validation of a general index of food craving, Appetite 49 (2007) 38–46, doi: 10.1016/j.appet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [51].Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH, Fifty years of the barratt impulsiveness scale: an update and review, Personal. Individ. Differ. 47 (2009) 385–395, doi: 10.1016/j.paid.2009.04.008. [DOI] [Google Scholar]
- [52].Watson D, Clark LA, Tellegen A, Development and validation of brief measures of positive and negative affect: the PANAS scales, J. Pers. Soc. Psychol 54 (1988) 1063–1070. [DOI] [PubMed] [Google Scholar]
- [53].Radloff LS, The CES-D scale: a self-report depression scale for research in the general population, Appl. Psychol. Assesment 1 (1977) 385–401. [Google Scholar]
- [54].Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, A scale for the assessment of hedonic tone the Snaith–Hamilton pleasure scale, Br. J. Psychiatry 167 (1995) 99–103, doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- [55].Lang PJ, Bradley MM, Cuthbert BN, International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual, University of Florida, Gainesville, FL, 2008. [Google Scholar]
- [56].Lam Versace CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM, Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long-term smoking abstinence: ERPs predict smoking cessation, Addict. Biol 17 (2012) 991–1000, doi: 10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Torrubia R, Ávila C, Moltó J, Caseras X, The sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions, Personal Individ. Differ. 31 (2001) 837–862, doi: 10.1016/S0191-8869(00)00183-5. [DOI] [Google Scholar]
- [58].Colaizzi JM, Flagel SB, Joyner MA, Gearhardt AN, Stewart JL, Paulus MP, Mapping sign-tracking and goal-tracking onto human behaviors, Neurosci. Biobehav. Rev. 111 (2020) 84–94, doi: 10.1016/j.neubiorev.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H, A selective role for dopamine in stimulus–reward learning, Nature 469 (2011) 53–57, doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stice E, Yokum S, Voelker P, Relation of FTO to BOLD response to receipt and anticipated receipt of food and monetary reward, food images, and weight gain in healthy weight adolescents, Soc. Cogn. Affect. Neurosci (2019), doi: 10.1093/scan/nsz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Koshy Cherian A, Kucinski A, Pitchers K, Yegla B, Parikh V, Kim Y, Valuskova P, Gurnani S, Lindsley CW, Blakely RD, Sarter M, Unresponsive choline transporter as a trait neuromarker and a causal mediator of bottom-Up attentional biases, J. Neurosci. 37 (2017) 2947–2959, doi: 10.1523/JNEUROSCI.3499-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M, Cholinergic control over attention in rats prone to attribute incentive salience to reward cues, J. Neurosci. 33 (2013) 8321–8335, doi: 10.1523/jneurosci.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pitchers KK, Wood TR, Skrzynski CJ, Robinson TE, Sarter M, The ability for cocaine and cocaine-associated cues to compete for attention, Behav. Brain Res. 320 (2017) 302–315, doi: 10.1016/j.bbr.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Narayanan NS, Medial frontal ~4-Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion, J. Neurophysiol. 114 (2015) 1310–1320, doi: 10.1152/jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Versace F, Kypriotakis G, Neuroaffective profiles are associated with e-cigarette use, Neuroscience (2022), doi: 10.1101/2022.02.04.479183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Frank DW, Cinciripini PM, Deweese MM, Karam-Hage M, Kypriotakis G, Lerman C, Robinson JD, Tyndale RF, Vidrine DJ, Versace F, Toward precision medicine for smoking cessation: developing a neuroimaging-based classification algorithm to identify smokers at higher risk for relapse, Nicotine Tob. Res. (2019), doi: 10.1093/ntr/ntz211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].DeLaRosa BL, Spence JS, Didehbani N, Tillman GD, Motes MA, Bass C, Kraut MA, Hart J, Neurophysiology of threat processing bias in combat-related post-traumatic stress disorder, Hum. Brain Mapp. 41 (2020) 218–229, doi: 10.1002/hbm.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Klomjai W, Katz R, Lackmy-Vallée A, Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS), Ann. Phys. Rehabil. Med. 58 (2015) 208–213, doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- [69].George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE, Schwartz T, Sackeim HA, Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial, Arch. Gen. Psychiatry 67 (2010) 507, doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- [70].Hanlon CA, Dowdle LT, Gibson NB, Li X, Hamilton S, Canterberry M, Hoffman M, Cortical substrates of cue-reactivity in multiple substance dependent populations: transdiagnostic relevance of the medial prefrontal cortex, Transl. Psychiatry 8 (2018), doi: 10.1038/s41398-018-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kearney-Ramos TE, Dowdle LT, Lench DH, Mithoefer OJ, Devries WH, George MS, Anton RF, Hanlon CA, Transdiagnostic effects of ventromedial prefrontal cortex transcranial magnetic stimulation on cue reactivity, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (2018) 599–609, doi: 10.1016/j.bpsc.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS, Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions, Cogn. Affect. Behav. Neurosci. 12 (2012) 241–268, doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


