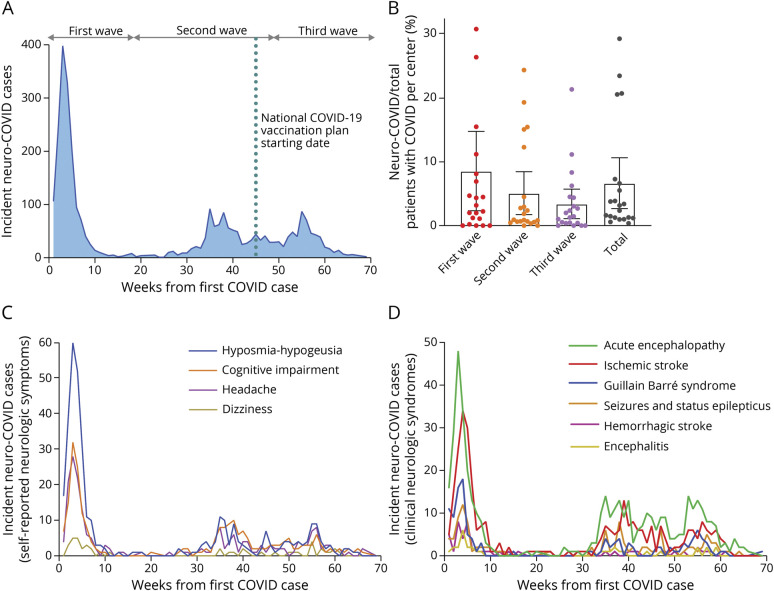
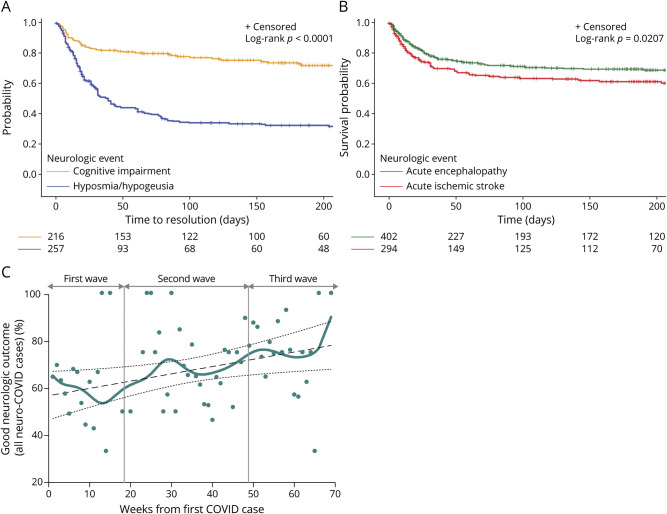
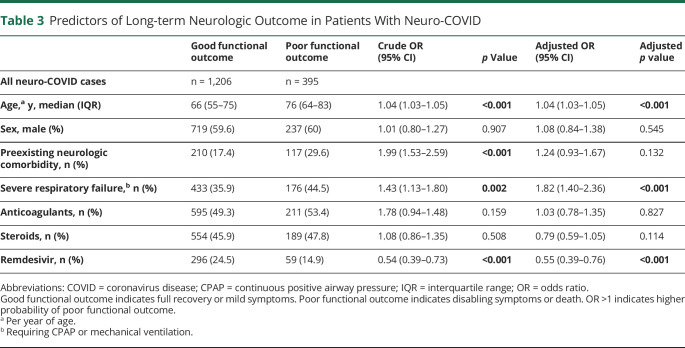
Simone Beretta
Simone Beretta, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,✉,
Viviana Cristillo
Viviana Cristillo, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Giorgia Camera
Giorgia Camera, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Carlo Morotti Colleoni
Carlo Morotti Colleoni, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Gaia Pellitteri
Gaia Pellitteri, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Beatrice Viti
Beatrice Viti, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Elisa Bianchi
Elisa Bianchi, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Stefano Gipponi
Stefano Gipponi, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Maria Grimoldi
Maria Grimoldi, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Mariarosaria Valente
Mariarosaria Valente, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Susanna Guttmann
Susanna Guttmann, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Maria Sofia Cotelli
Maria Sofia Cotelli, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Pasquale Palumbo
Pasquale Palumbo, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Giorgio Gelosa
Giorgio Gelosa, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Stefano Meletti
Stefano Meletti, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Cristina Schenone
Cristina Schenone, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Donatella Ottaviani
Donatella Ottaviani, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Massimo Filippi
Massimo Filippi, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Andrea Zini
Andrea Zini, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Paola Basilico
Paola Basilico, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Lucia Tancredi
Lucia Tancredi, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Pietro Cortelli
Pietro Cortelli, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Massimiliano Braga
Massimiliano Braga, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Valeria De Giuli
Valeria De Giuli, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Serenella Servidei
Serenella Servidei, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Damiano Paolicelli
Damiano Paolicelli, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Federico Verde
Federico Verde, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Stefano Caproni
Stefano Caproni, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Antonio Pisani
Antonio Pisani, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Vincenzina Lo Re
Vincenzina Lo Re, MD, FEBN
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Luca Massacesi
Luca Massacesi, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Daria Valeria Roccatagliata
Daria Valeria Roccatagliata, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Paolo Manganotti
Paolo Manganotti, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Daniele Spitaleri
Daniele Spitaleri, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Anna Formenti
Anna Formenti, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Marta Piccoli
Marta Piccoli, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Silvia Marino
Silvia Marino, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Paola Polverino
Paola Polverino, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Umberto Aguglia
Umberto Aguglia, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Raffaele Ornello
Raffaele Ornello, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Elisabetta Perego
Elisabetta Perego, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Gabriele Siciliano
Gabriele Siciliano, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Paola Merlo
Paola Merlo, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Marco Capobianco
Marco Capobianco, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Leonardo Pantoni
Leonardo Pantoni, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Alessandra Lugaresi
Alessandra Lugaresi, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Stefania Angelocola
Stefania Angelocola, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Anna De Rosa
Anna De Rosa, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Maria Sessa
Maria Sessa, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Ettore Beghi
Ettore Beghi, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Elio Clemente Agostoni
Elio Clemente Agostoni, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Salvatore Monaco
Salvatore Monaco, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Alessandro Padovani
Alessandro Padovani, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Alberto Priori
Alberto Priori, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Vincenzo Silani
Vincenzo Silani, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Gioacchino Tedeschi
Gioacchino Tedeschi, MD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1,
Carlo Ferrarese
Carlo Ferrarese, MD, PhD
1From the Department of Neurology (S.B., C.M.C., C.F.), Fondazione IRCCS San Gerardo dei Tintori, Monza; Department of Medicine and Surgery (S.B., C.M.C., C.F.), University of Milano Bicocca; The Milan Center for Neuroscience (NeuroMI) (S.B., G.G., C.F.); Neurology Unit and Department of Clinical and Experimental Sciences (V.C., S. Gipponi, A. Padovani), University of Brescia; Unit of Neurology and Neurophysiology (G.C., M.G., M.S.), ASST PG23, Bergamo; Santa Maria della Misericordia University Hospital (G.P., M.V.), Udine, Italy; San Marino Neurological Unit (B.V., S. Guttmann), San Marino Hospital; The Mario Negri Institute for Pharmacological Research IRCCS (E. Bianchi, E. Beghi), Milan; Department of Medical Area (DAME) (M.V.), University of Udine; Neurology Unit (M.S.C.), ASST Valcamonica, Esine, Brescia; USL Centro Toscana (P. Palumbo), Neurology Unit, Nuovo Ospedale Santo Stefano, Prato; Department of Neurology and Stroke Unit (G.G., E.C.A.), Niguarda, Milan; Department of Neurology and Department of Clinical Neurophysiology AOU Modena (S. Meletti), University of Modena and Reggio Emilia; Department of Neuroscience, Rehabilitation, Ophtalmology, Genetics, Maternal and Child Health (C.S.), University of Genoa; Ospedale Santa Maria del Carmine di Rovereto (D.O.), Trento; Neurology Unit (M.F.), IRCCS San Raffaele Scientific Institute, Milan; Department of Neurology (A.Z.), Metropolitan Stroke Network, Ospedale Maggiore, Bologna; Department of Neurology (P.B.), Ospedale A. Manzoni ASST Lecco; University of Milan (L.T., L.P., A. Priori); Neurology Unit (L.T., A. Priori), ASST Santi Paolo e Carlo; Aldo Ravelli Center for Neurotechnology and Experimental Brain Therapeutics (L.T.), Milan; IRCCS Institute of Neurological Science of Bologna (P.C.); DIBINEM (P.C.), University of Bologna; UOC Neurology (M.B.), ASST Vimercate; Department of Neurology (V.D.G.), ASST Cremona; Neurophysiopathology Unit, Fondazione Policlinico Universitario A. Gemelli, Rome, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs (D.P.), University of Bari; Department of Neurology and Laboratory of Neuroscience (F.V., V.S.), IRCCS Istituto Auxologico Italiano; “Dino Ferrari” Center (F.V., V.S.), Department of Pathophysiology and Transplantation, Università degli Studi di Milano; Neurology Division (S.C.), “S. Maria” University Hospital, Terni; IRCCS Mondino Foundation (A. Pisani), Department of Brain and Behavioral Sciences, University of Pavia; Department of Diagnostic and Therapeutic Services (V.L.R.), IRCCS ISMETT, Palermo; Department of Neurology 2 (L.M.), Careggi University Hospital, Florence; Department of Neurology and Neurosurgery (D.V.R.), ASST di Mantova; Clinical Neurology Unit (P. Manganotti), Cattinara University Hospital, University of Trieste; Department of Neurology (D.L.A.S.), AORN S.Giovanni Moscati, Avellino; Neurology and Stroke Unit (A.F.), Neuroscience Department, ASST-Lecco, Merate; Department of Neurology (M.P.), Ospedale San Filippo Neri, Rome; IRCCS Centro Neurolesi Bonino-Pulejo (S. Marino), Messina; Department of Neurology (P. Polverino), IRCCS Humanitas Research Hospital, Rozzano, Milan; Department of Medical and Surgical Sciences (U.A.), Magna Graecia University of Catanzaro; Department of Biotechnological and Clinical Sciences (R.O.), University of L'Aquila; Department of Neurology (E.P.), Ospedale Valduce, Como; Neurological Clinic (G.S.), University of Pisa; Department of Neurology (P. Merlo), Humanitas Gavazzeni, Bergamo; Department of Neurology (M.C.), S. Luigi Gonzaga Hospital, Orbassano; Ospedale Luigi Sacco (L.P.), Milan; IRCCS Institute of Neurological Science of Bologna (A.L.), UOSI Multiple Sclerosis Rehabilitation; Department of Biomedical Science and Neuromotricity (A.L.), University of Bologna; Department of Neurology (S.A.), Fermo; Department of Neurosciences (A.D.R.), Federico II University, Naples; Neurology Unit and Department of Neurosciences (S. Monaco), University of Verona; IRCCS Fondazione Ospedale Maggiore Policlinico (A. Priori), Milan; and Department of Advanced Medical and Surgical Sciences (G.T.), University of Campania, Naples, Italy.
1;
for Neuro-COVID Italy1