Abstract
Background and Objectives
There is growing evidence for endovascular thrombectomy (EVT) in patients with large ischemic core infarct and large vessel occlusion (LVO). The objective of this study was to compare the efficacy and safety of EVT vs medical management (MM) using a systematic review and meta-analysis of observational studies and randomized controlled trials (RCTs).
Methods
We searched the PubMed, Embase, Cochrane Library, and Web of Science databases to obtain articles related to mechanical thrombectomy for large ischemic core from inception until February 10, 2023. The primary outcome was independent ambulation (modified Rankin Scale [mRS] 0–3). Effect sizes were computed as risk ratio (RR) with random-effect or fixed-effect models. The quality of articles was evaluated through the Cochrane risk assessment tool and the Newcastle-Ottawa Scale. This study was registered in PROSPERO (CRD42023396232).
Results
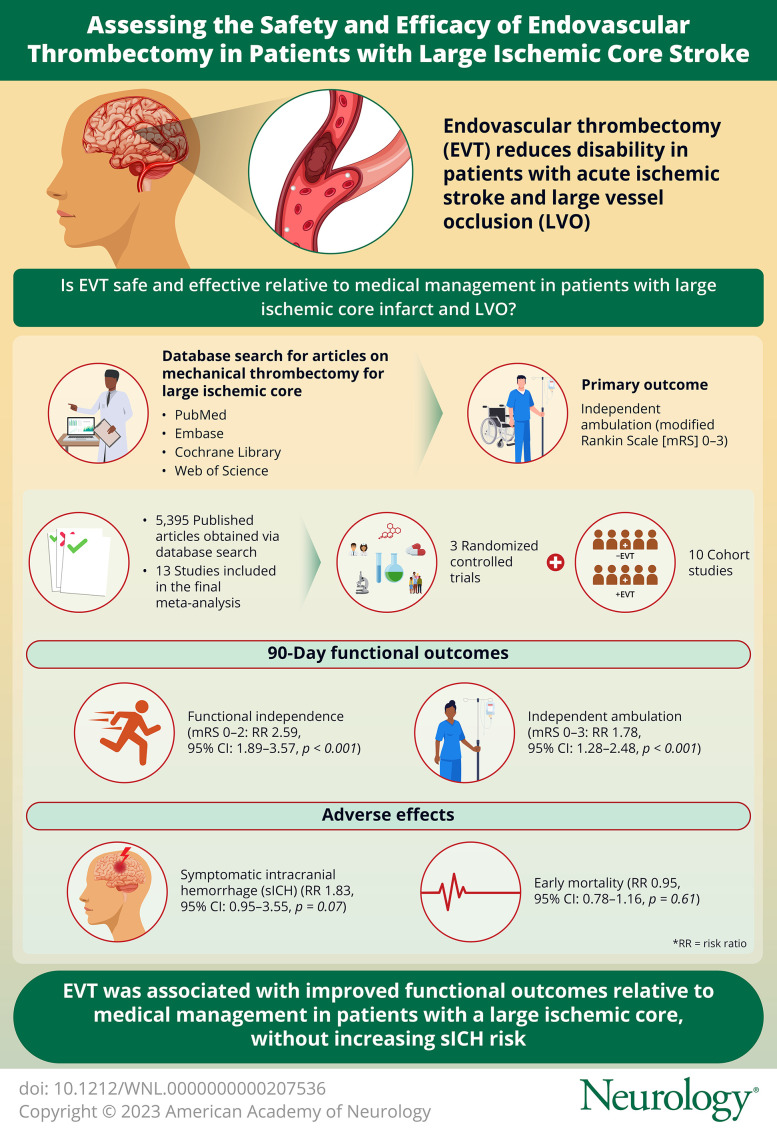
A total of 5,395 articles were obtained through the search and articles that did not meet the inclusion criteria were excluded by review of the title, abstract, and full text. Finally, 3 RCTs and 10 cohort studies met the inclusion criteria. The RCT analysis showed that EVT improved the 90-day functional outcomes of patients with large ischemic core with high-quality evidence, including independent ambulation (mRS 0–3: RR 1.78, 95% CI 1.28–2.48, p < 0.001) and functional independence (mRS 0–2: RR 2.59, 95% CI 1.89–3.57, p < 0.001), but without significantly increasing the risk of symptomatic intracranial hemorrhage (sICH: RR 1.83, 95% CI 0.95–3.55, p = 0.07) or early mortality (RR 0.95, 95% CI 0.78–1.16, p = 0.61). Analysis of the cohort studies showed that EVT improved functional outcomes of patients without an increase in the incidence in sICH.
Discussion
This systematic review and meta-analysis indicates that in patients with LVO stroke with a large ischemic core, EVT was associated with improved functional outcomes over MM without increasing sICH risk. The results of ongoing RCTs may provide further insight in this patient population.
Introduction
Several randomized controlled trials (RCTs) have indicated that endovascular thrombectomy (EVT) reduces disability of patients with acute ischemic stroke and large vessel occlusion (LVO).1-8 The approach has achieved level 1a recommendation for select patients in stroke guidelines.9-13 However, most of these trials had strict imaging inclusion criteria, recruiting patients with a baseline Alberta Stroke Program Early CT Score (ASPECTS) greater than 5 on noncontrast CT or with an infarct core volume less than 70 mL on CT perfusion.3,4,6,14 Patients with a large infarct core (volume greater than 70 mL, ASPECTS score of 5 or less) who were believed to be less likely to benefit from thrombectomy and at increased risk of reperfusion injury or symptomatic intracranial hemorrhage (sICH) were excluded from the early EVT trials.7,9,15-17 Therefore, the efficacy of EVT in patients with a larger ischemic burden has not been well studied.
The Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) individual patient-level meta-analysis of the thrombectomy trials compared the effect of mechanical thrombectomy vs medical therapy across different strata of patients with small, medium, or large infarct core.18 A subgroup analysis of 126 patients with ASPECTS 0 to 4 showed that the point estimates favored thrombectomy compared with medical management (MM) for the primary outcome of neurological disability (90-day ordinal modified Rankin Scale [mRS]), however with a higher rate of sICH (EVT vs MM: 19% vs 5%, p = 0.016). A prior meta-analysis of 9 observational studies including 1,196 patients with LVO and low ASPECTS (≤5) who received mechanical thrombectomy showed that favorable outcome could be achieved despite a trend of higher sICH.19 The Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism–Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT) showed that patients with large infarct core achieved better functional outcomes with mechanical thrombectomy than with medical therapy alone.20 A similar treatment effect was reported in 2 other randomized trials, the Randomized Controlled Trial to Optimize Patient's Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT2), and the Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients with a Large Infarct Core (ANGEL-ASPECT).21,22
This systematic review and meta-analysis aims to provide an updated summary of the relevant literature, combining the pooled results of 3 RCTs (RESCUE-Japan LIMIT, SELECT2, and ANGEL-ASPECT) and observational cohort studies, to investigate the efficacy and safety of mechanical thrombectomy in acute large ischemic stroke across a diverse population.
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines.23 This study protocol was registered on the International Prospective Register of Systematic Reviews on February 12, 2023 (PROSPERO, CRD42023396232). Data are available on request to the corresponding authors.
Data Source and Search Strategy
We searched the PubMed, Embase, Cochrane Library, and Web of Science databases to obtain articles in all languages from inception until February 10, 2023. “Stroke,” “Thrombectomy,” and “large ischemic core” were the search terms. Synonyms were obtained from PubMed, Embase, and Cochrane Library with elimination of duplicates. Detailed search criteria of keywords and their synonyms are provided in eTable 1 (links.lww.com/WNL/C859).
Eligibility Criteria
The inclusion criteria for this large ischemic core systematic review and meta-analysis were as follows: (1) patients with ASPECTS ≤5 or infarct core volume ≥50 mL, (2) RCT or observational study, (3) interventional arm receiving EVT and MM, (4) control arm receiving MM, and (5) reporting of mRS score of 0–3 at 3 months, 90-day mortality, and sICH. Studies were excluded if it lacked report of the primary study outcomes or if it lacked reporting of a control group. Randomized trials with less than 100 patients were excluded.
Study Selection and Data Collection
The titles, abstracts, and full texts of the articles were read by 2 researchers working independently (Q.L., Y.D.), selected according to the inclusion and exclusion criteria from a predesigned table as detailed in eTable 2 (links.lww.com/WNL/C859). The 2 researchers conducted cross-checking after screening of the articles, and if there was disagreement, it was resolved through discussion with the senior author (Z.Q.). Data of the baseline characteristics, primary, secondary, and safety end points of each study were extracted for analysis by 2 researchers independently (Q.L., Y.D.).
Risk of Bias Assessment and Quality of Evidence
The quality of the RCTs and risk of bias were evaluated with the Cochrane risk assessment tool. The cohort and case-control studies were evaluated by the Newcastle-Ottawa Scale (NOS). For retrospective studies, an evaluation result ≥5 ☆ was considered of good quality and was included in the meta-analysis. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used to evaluate the overall quality of evidence. Publication bias was examined by funnel plot.
Effect Measures
The primary outcome was independent ambulation (defined as a mRS score of 0–3) at 90 days. The secondary outcomes were functional independence (mRS 0–2) and the rate of decompressive hemicraniectomy. The safety outcomes were sICH defined according to study criteria and mortality at 90 days.
Statistical Analysis
Statistical analysis was performed using RevMan5.4 and Stata Software (version 12.0). For the RCTs, data were reported as intention-to-treat analysis. Absolute counts are provided in addition to effect estimates, which are expressed as risk ratios (RR) with corresponding 95% CIs. The χ2 test was used to analyze the heterogeneity of the results in each study. It was considered that an I2 < 50% and p > 0.1 indicated that the combined results were homogeneous, and hence, the fixed-effect model was used for analysis. When I2 was ≥50% or p ≤ 0.1, this indicated that the combination result had heterogeneity, and therefore, the random-effect model was used to analyze and search for possible sources of heterogeneity.
Standard Protocol Approvals, Registrations, and Patient Consents
This systematic review and meta-analysis was registered prospectively PROSPERO on February 12, 2023, which used summary data from published manuscripts (crd.york.ac.uk/prospero/display_record.php?ID=CRD42023396232). We did not use individual level data, so informed consent or IRB approval was not required.
Data Availability
Data not provided in the article because of space limitations may be shared at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Study Characteristics and Quality Evaluation
A total of 5,395 articles were obtained through search and articles that did not meet the inclusion criteria were excluded by reading the title, abstract, and full text (Figure 1). Finally, 3 RCTs and 10 observational cohort studies met the inclusion criteria, with basic characteristics shown in Tables 1 and 2, respectively (other baseline data are presented in eTable 3 and eTable 4, links.lww.com/WNL/C859). The 3 RCTs were at low risk of bias (Table 1, eFigure 1). Ten cohort studies were scored with the NOS, with a score of 8☆ to 9☆, thus meeting the conditions for inclusion in this meta-analysis (Table 1, eTable 5). A total of 2,861 patients were included in this analysis. The RCTs included 1,010 patients, of whom 509 patients were treated with EVT and 501 treated with MM. The cohort study included 1,851 patients of whom 879 patients were treated with EVT and 972 treated with MM.
Figure 1. PRISMA Flow Diagram.
PRISMA = Preferred Reporting Items for Systematic Review and Meta-Analysis; RCT = randomized controlled trial.
Table 1.
Characteristics and Quality Evaluation of Randomized Controlled Trials
Table 2.
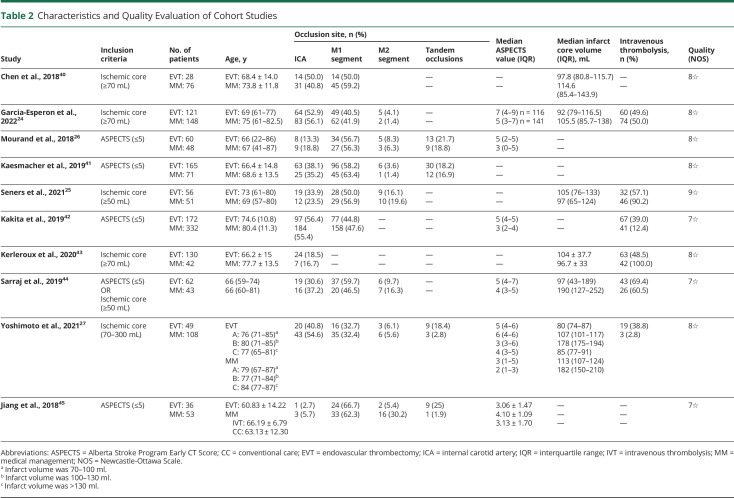
Characteristics and Quality Evaluation of Cohort Studies
Primary Outcome: Independent Ambulation (mRS 0–3)
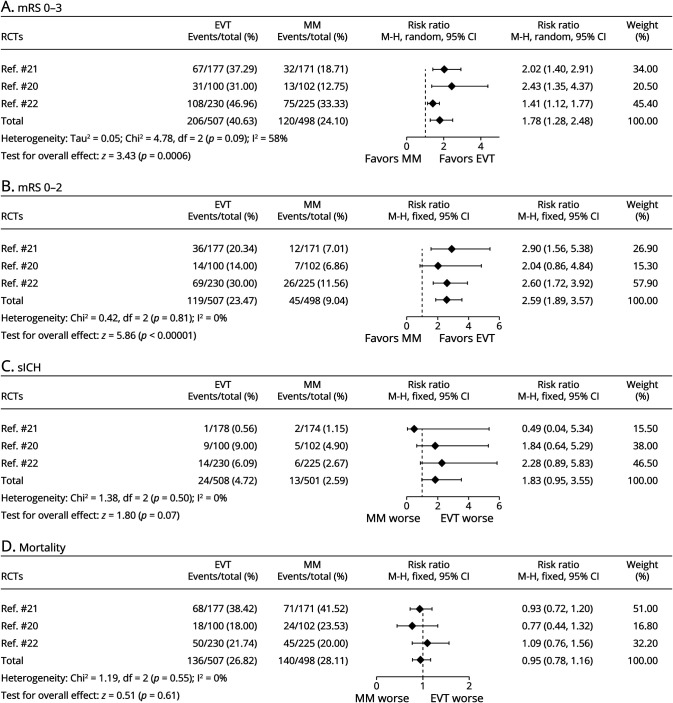
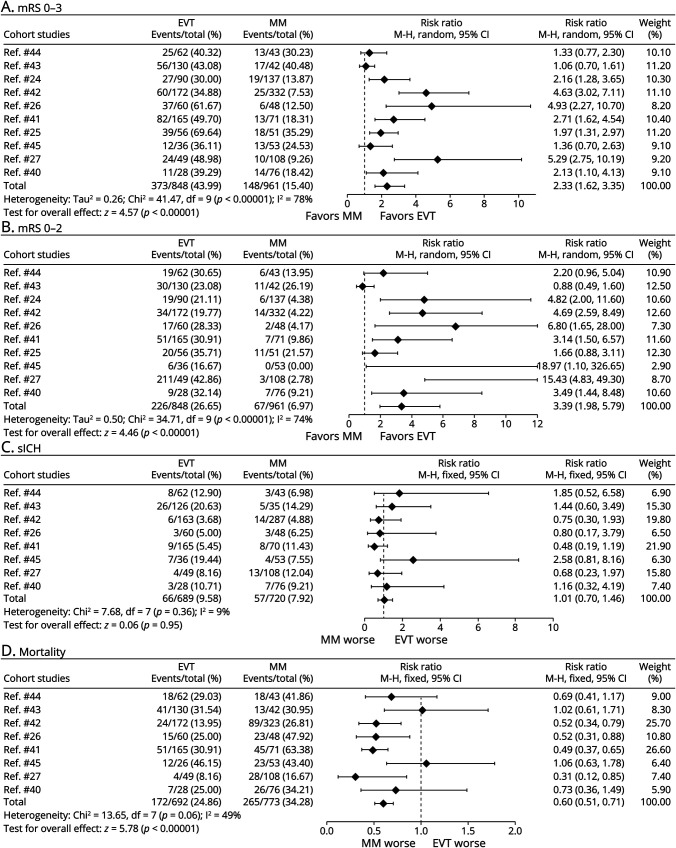
Three RCTs and 10 observational cohort studies were combined and analyzed using the random-effect model (Figures 2A and 3A, respectively). Across the 3 RCTs, EVT improved the primary outcome of independent ambulation in patients with large ischemic core (RR 1.78, 95% CI 1.28–2.48, p < 0.001) and showed moderate heterogeneity (I2 = 58%). In 10 observational cohort studies, the same conclusion was reached (RR 2.33, 95% CI 1.62–3.35, p < 0.001), but exhibited high heterogeneity (I2 = 78%). The GRADE quality of the RCT evidence was high whereas the GRADE quality of the cohort studies was low (eFigure 2, links.lww.com/WNL/C859).
Figure 2. Forest Plots of RCTs About (A) Independent Ambulation (mRS ≤3), (B) Functional Independence (mRS ≤2), (C) Incidence of sICH (per Study Definition), and (D) Mortality.
EVT = endovascular thrombectomy; MM = medical management; mRS = modified Rankin Scale; RCT = randomized controlled trial; sICH = symptomatic intracranial hemorrhage.
Figure 3. Forest Plots of Observational Cohort Studies About (A) Independent Ambulation (mRS ≤3), (B) Functional Independence (mRS ≤2), (C) Incidence of sICH, and (D) Mortality.
EVT = endovascular thrombectomy; MM = medical management; mRS = modified Rankin Scale; RCT = randomized controlled trial; sICH = symptomatic intracranial hemorrhage.
Secondary Outcome: Functional Independence (mRS 0–2)
The fixed-effect model was used to combine the results of the 3 RCTs (Figure 2B) and showed that for patients with large ischemic core, EVT improved the likelihood of functional independence (RR 2.59, 95% CI 1.89–3.57, p < 0.001), with very low heterogeneity (I2 = 0%). The same results were obtained after pooling 10 cohort studies with the random-effect model (RR 3.39, 95% CI 1.98–5.79, p < 0.001) (Figure 3B), but the heterogeneity was high (I2 = 74%). The GRADE quality of evidence for the 3 RCTs was high, and the GRADE quality of evidence for the observational cohort studies was low (eFigure 2, links.lww.com/WNL/C859).
Symptomatic Intracranial Hemorrhage
In the RCT analysis, there was a numerically higher rate of sICH in the EVT compared with the MM group; however, this was not significant (24/508, 4.7% vs 13/501, 2.6%, p = 0.07). Owing to the very low heterogeneity (I2 = 0%), the fixed-effect model was used to combine sICH data from the RCTs. The probability of sICH in patients with large infarct region treated with EVT was 1.83 times higher than that in patients treated with MM (RR 1.83, 95% CI 0.95–3.55, p = 0.07), also not significant (Figure 2C). Analysis of 8 cohort studies showed that the probability of sICH was similar between EVT and MM (RR 1.01, 95% CI 0.70–1.46; p = 0.95) (Figure 3C). Two of the cohort studies did not include sICH data and were not included in the sICH analysis.24,25 The GRADE quality of the RCT evidence was high and that of the cohort studies was very low (eFigure 2, links.lww.com/WNL/C859).
Decompressive Hemicraniectomy
Two RCTs20,22 and 2 cohort studies26,27 reported data on decompressive craniectomy (DC). Analysis of the 2 RCTs showed that there was no difference in the rate of DC between the EVT and MM group (RR 1.22, 95% CI 0.43–3.41), whereas the cohort study showed that EVT was associated with a lower rate of DC (RR 0.21, 95% CI 0.07–0.59, eFigure 3, links.lww.com/WNL/C859).
Mortality
Three RCTs and 8 cohort studies were combined and analyzed using the fixed-effect model. In the 3 RCTs, there was no increased mortality in the EVT group compared with the MM group (RR 0.95, 95% CI 0.78–1.16, p = 0.61) (Figure 2D), showing an extremely low heterogeneity (I2 = 0%). In 8 observational cohort studies, EVT reduced mortality of patients with large ischemic core (RR 0.60, 95% CI 0.51–0.71, p < 0.001) (Figure 3D), and there was a moderate heterogeneity (I2 = 49%). As 2 of the cohort studies did not include mortality data, they were not included in the mortality analysis.24,25 The GRADE quality of the RCT evidence was moderate and that of the cohort studies was very low (eFigure 2, links.lww.com/WNL/C859).
Risk of Bias
A funnel plot of the 3 RCTs was symmetric indicating that there was no publication bias (Egger test p > 0.05, eFigure 4, links.lww.com/WNL/C859).
Discussion
In the present systematic review and meta-analysis comprising patients of diverse international representation with anterior circulation LVO and large ischemic core infarct, we found from the analysis of 3 pooled RCTs that patients who underwent EVT had a nearly twofold higher chance of independent ambulation at 90 days, over twofold higher probability of achieving 90-day functional independence, a numerically higher rate of sICH, and comparable mortality rate when compared with standard care alone. These treatment effects were generally concordant with the 10 observational studies except that mortality was noted to be lower in the EVT group from the observational studies.
The pooled treatment effect favoring EVT over MM across these 3 large ischemic core randomized trials is less than that observed in the HERMES meta-analysis comprising patients with small core infarct (adjusted common odds 2.49, 95% CI 1.76–3.53; p < 0.0001). HERMES selected ordinal mRS shift as the primary outcome, while we selected the primary outcome as independent ambulation as defined by mRS 0–3.5 As patients with large ischemic stroke have more extensive infarction at baseline, a clinically meaningful outcome for these patients can be widened to mRS 0–3, which translates to requiring some help, but the patient is independent with ambulation.16,28 With data from these 3 RCTs, our findings now lend support that EVT confers benefit across a broader strata of patients with moderate-sized to large-sized infarcts in an up to 24 hours window from time last known well. As such, advanced imaging modalities to triage patients with LVO in the 6–24 hour window may no longer be necessary to select patients with widening of thrombectomy eligibility criteria to include patients with large ischemic core.12,29,30 However, as patients with very large infarct core as defined by ASPECTS 0–2 were excluded from RESCUE-Japan LIMIT and under-represented in ANGEL-ASPECT and SELECT2, we could not establish the benefit of EVT in this subgroup of patients with very large ischemic core.
In the pooled analysis of independent ambulation, the 3 RCTs showed moderate heterogeneity, while the heterogeneity decreased to 0% after removing ANGEL-ASPECT but only combining the results of SELECT2 and RESCUE-Japan LIMIT (eFigure 5, links.lww.com/WNL/C859). We surmise that this may be due to the fact that the median infarct core volume of patients included in ANGEL-ASPECT was smaller than that of the other 2 trials (Table 1). Subgroup analysis of SELECT2 also showed that patients with larger infarction volume (≥100 mL) were likely to benefit from EVT.21 Similarly, subgroup analysis of patients with larger infarct core volume (>70 mL) in ANGEL-ASPECT also suggested the point estimates in favor of EVT, although this did not achieve significance.
With regard to adverse events, the RCTs showed that the sICH rate of patients with large ischemic core treated with EVT was 1.83 times higher than that of MM, whereas there was no difference between the EVT and MM groups with regard to sICH from the observational cohort studies. This may be due to the variable criteria for evaluating sICH across different studies, such as use of the Heidelberg bleeding classification in ANGEL-ASPECT or Safe Implementation of Thrombolysis in Stroke-Monitoring Study in RESCUE-Japan LIMIT and SELECT2. Moreover, the overall rate of sICH was surprisingly low across all 3 randomized trials (EVT vs MM: 4.7% vs 2.6%) whereas the overall rates of sICH were more than twofold higher across the 8 observational cohorts (EVT vs MM 9.5% vs 7.9%). Perhaps the rigor by which patients are being managed in RCTs may, in part, explain safety differences between trial and real-world patients.31,32
Regarding mortality in patients with large ischemic core, EVT did not increase mortality in the RCT analysis, while cohort studies showed that EVT significantly reduced mortality. From the distribution plot of mRS scores of RCTs, the difference in mortality between the EVT group and the standard medical therapy group of the 3 RCTs was not significant, but the proportion of mRS 5–6 between the 2 groups was significantly different (SELECT2: EVT vs MM = 46.6% vs 59.2%; RESCUE-Japan LIMIT: EVT vs MM = 36% vs 64%; ANGEL-ASPECT: EVT vs MM = 33.4% vs 40%).20-22 The above findings indicate that although EVT was not shown to reduce mortality and has a numerically higher risk of sICH, it can significantly reduce severe disability. Altogether, EVT, as a minimally invasive neurological procedure, can significantly improve the functional outcomes, and the incidence of adverse events is acceptable given the natural history of this condition.
We also analyzed the impact of DC on survival and functional outcomes in patients with large core infarction. Lack of a difference in the rates of DC between the EVT and MM groups in the RCT analysis was surprising considering that prior cohort or nation-wide analyses showed that EVT was associated with lower rates of DC.33,34 It is possible the RCT analysis was underpowered to detect a potential difference. Moreover, there was no difference in preoperative clinical parameters, ASPECTS, and clinical outcome between DC patients who were treated with EVT and those who were not in a retrospective study.35 In addition, the success of EVT (complete or near complete reperfusion) did not obviate need for DC.35 In summary, EVT did not change the rate of subsequent DC in the RCT analysis.
Our study has limitations. First, the number of RCTs was limited. When evaluating the primary outcome for independent ambulation, the 3 RCTs had a low-to-moderate degree of heterogeneity whereas the cohort studies had moderate-to-severe heterogeneity. Second, there was wide variation in the imaging modality choice and definition of imaging parameters across these large ischemic core trials. There remains controversy in defining a true large ischemic core infarction in current clinical practice. Not all ASPECTS regions have the same infarct volume, which means that when ASPECTS ≤5 is the only criterion, patients with infarct core volume smaller than 50 mL may also be included. Third, owing to the lack of data on subgroup analysis, we did not identify the benefit of EVT and risk ratio of patients with very large ischemic core (i.e., ASPECTS 0–2) or as stratified by age.36 Some studies have suggested that patients with ischemic core ≥130 mL do not benefit27 or are at risk of increased edema after reperfusion.37 However, in SELECT-2, even patients with >150 mL ischemic core seemed to benefit, and further analyses may be warranted.21
This systematic review and meta-analysis indicated that EVT is associated with better functional outcomes than standard medical therapy in acute LVO stroke with a large ischemic core. Less restrictive patient selection criteria lead to treatment benefit. The results of 3 additional RCTs, TESLA (ClinicalTrials.gov, NCT03805308),38 TENSION (ClinicalTrials.gov, NCT03094715), and LASTE (ClinicalTrials.gov, NCT03811769) will provide further guidance on the management of these patients.39 A meta-analysis of individual patient data is expected.
Glossary
- ANGEL-ASPECT
Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients with a Large Infarct Core
- ASPECTS
Alberta Stroke Program Early CT Score
- DC
decompressive craniectomy
- EVT
endovascular thrombectomy
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HERMES
Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials
- LVO
large vessel occlusion
- MM
medical management
- mRS
modified Rankin Scale
- NOS
Newcastle-Ottawa Scale
- RESCUE-Japan LIMIT
Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism–Japan Large Ischemic Core Trial
- RCT
randomized controlled trial
- RR
risk ratio
- SELECT2
Randomized Controlled Trial to Optimize Patient's Selection for Endovascular Treatment in Acute Ischemic Stroke
- sICH
symptomatic intracranial hemorrhage
Appendix. Authors
Footnotes
Infographic: links.lww.com/WNL/D14
Study Funding
No targeted funding reported.
Disclosure
J. Kaesmacher is supported by the grants from the Swiss Academy of Medical Sciences/Bangerter Foundation, Swiss Stroke Society, Clinical Trials Unit Bern, and the Swiss National Science Foundation. A. Sarraj is Principal Investigator of the SELECT2 trial funding by grants from Stryker Neurovascular to University of Texas-McGovern Medical School and University Hospitals—Cleveland Medical Center. J.L. Saver has received contracts from Medtronic, Abbott, NeuroVasc, Phillips Medical, Bayer, Biogen, Roche, BrainsGate, BrainQ, and Occlutech and stock options from Rapid Medical and QuantalX for service on clinical trial steering committees and Data and Security Monitoring Committees and advising on rigorous study design and conduct. T.N. Nguyen received research support from Medtronic and served on advisory board of Idorsia. R.G. Nogueira reports consulting fees from Anaconda, Biogen, Cerenovus, Genentech, Philips, Hybernia, Imperative Care, Medtronic, Phenox, Philips, Prolong Pharmaceuticals, Stryker Neurovascular, Shanghai Wallaby and Synchron and stock options from Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, Vesalio, Viz-AI, RapidPulse, and Perfuze for advisory serve; is the Principal Investigator of the “Combined Thrombectomy for Distal MediUm Vessel Occlusion StroKe (DUSK)” trial funding by Stryker Neurovascular; and is an Investor in Viz-AI, Perfuze, Cerebrotech, Reist/Q'Apel Medical, Truvic, Vastrax, and Viseon. A.J. Yoo and O.O. Zaidat report PI of the Thrombectomy for Emergent Salvage of Large Anterior Ischemic Stroke (TESLA) trial. The remaining authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/nejmoa1415061 [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/nejmoa1411587 [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/nejmoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/nejmoa1706442 [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/s0140-6736(16)00163-x [DOI] [PubMed] [Google Scholar]
- 6.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/nejmoa1503780 [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/nejmoa1414905 [DOI] [PubMed] [Google Scholar]
- 8.Martins SO, Mont'Alverne F, Rebello LC, et al. Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382(24):2316-2326. doi: 10.1056/nejmoa2000120 [DOI] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/str.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 10.Yamagami H, Hayakawa M, Inoue M, et al. Guidelines for mechanical thrombectomy in Japan, the Fourth Edition, March 2020: a guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir. 2021;61(3):163-192. doi: 10.2176/nmc.nmc.st.2020-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO): European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TN, Castonguay AC, Siegler JE, et al. Mechanical Thrombectomy in the Late Presentation of Anterior Circulation Large Vessel Occlusion Stroke: A Guideline From the Society of Vascular and Interventional Neurology Guidelines and Practice Standards Committee. Stroke: Vascular and Interventional Neurology 2023;3:e000512. [Google Scholar]
- 13.Eskey CJ, Meyers PM, Nguyen TN, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation. 2018;137(21):e661-e689. doi: 10.1161/cir.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 14.Abdalkader M, Siegler JE, Lee JS, et al. Neuroimaging of acute ischemic stroke: multimodal imaging approach for acute endovascular therapy. J Stroke. 2023;25(1):55-71. doi: 10.5853/jos.2022.03286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TN, Raymond J, Nogueira RG, Fischer U, Siegler JE. The problem of restrictive thrombectomy trial eligibility criteria. Stroke. 2022;53(9):2988-2990. doi: 10.1161/strokeaha.122.040006 [DOI] [PubMed] [Google Scholar]
- 16.Fayad P. Improved prospects for thrombectomy in large ischemic stroke. N Engl J Med. 2023;388(14):1326-1328. doi: 10.1056/NEJMe2300193 [DOI] [PubMed] [Google Scholar]
- 17.Fahed R, Finitsis S, Khoury N, et al. A randomized pragmatic care trial on endovascular acute stroke interventions (EASI): criticisms, responses, and ethics of integrating research and clinical care. Trials. 2018;19(1):508. doi: 10.1186/s13063-018-2870-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Román LS, Menon BK, Blasco J, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17(10):895-904. doi: 10.1016/s1474-4422(18)30242-4 [DOI] [PubMed] [Google Scholar]
- 19.Diestro JDB, Dmytriw AA, Broocks G, et al. Endovascular thrombectomy for low ASPECTS large vessel occlusion ischemic stroke: a systematic review and meta-analysis. Can J Neurol Sci. 2020;47(5):612-619. doi: 10.1017/cjn.2020.71 [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386(14):1303-1313. doi: 10.1056/nejmoa2118191 [DOI] [PubMed] [Google Scholar]
- 21.Sarraj A, Hassan AE, Abraham MG, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388(14):1259-1271. doi: 10.1056/NEJMoa2214403 [DOI] [PubMed] [Google Scholar]
- 22.Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388(14):1272-1283. doi: 10.1056/NEJMoa2213379 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Esperon C, Bivard A, Johns H, et al. Association of endovascular thrombectomy with functional outcome in patients with acute stroke with a large ischemic core. Neurology. 2022;99(13):e1345-e1355. doi: 10.1212/wnl.0000000000200908 [DOI] [PubMed] [Google Scholar]
- 25.Seners P, Oppenheim C, Turc G, et al. Perfusion imaging and clinical outcome in acute ischemic stroke with large core. Ann Neurol. 2021;90(3):417-427. doi: 10.1002/ana.26152 [DOI] [PubMed] [Google Scholar]
- 26.Mourand I, Abergel E, Mantilla D, et al. Favorable revascularization therapy in patients with ASPECTS ≤ 5 on DWI in anterior circulation stroke. J Neurointerv Surg. 2018;10(1):5-9. doi: 10.1136/neurintsurg-2017-013358 [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto T, Inoue M, Tanaka K, et al. Identifying large ischemic core volume ranges in acute stroke that can benefit from mechanical thrombectomy. J Neurointerv Surg. 2021;13(12):1081-1087. doi: 10.1136/neurintsurg-2020-016934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darsaut TE, Collins J, Raymond J. Patients may be right: clinical research should be designed in their best medical interest. Neurochirurgie. 2023;69(1):101391. doi: 10.1016/j.neuchi.2022.101391 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen TN, Abdalkader M, Nagel S, et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol. 2022;79(1):22-31. doi: 10.1001/jamaneurol.2021.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seker F, Qureshi MM, Möhlenbruch MA, et al. Reperfusion without functional independence in late presentation of stroke with large vessel occlusion. Stroke. 2022;53(12):3594-3604. doi: 10.1161/strokeaha.122.039476 [DOI] [PubMed] [Google Scholar]
- 31.Tarlov N, Nien YL, Zaidat OO, Nguyen TN. Periprocedural management of acute ischemic stroke intervention. Neurology. 2012;79(13, suppl 1):S182-S191. doi: 10.1212/wnl.0b013e31826958d3 [DOI] [PubMed] [Google Scholar]
- 32.Prabhakaran S, Jovin TG, Tayal AH, et al. Posttreatment variables improve outcome prediction after intra-arterial therapy for acute ischemic stroke. Cerebrovasc Dis. 2014;37(5):356-363. doi: 10.1159/000362591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sporns PB, Minnerup J, Warneke N, et al. Impact of the implementation of thrombectomy with stent retrievers on the frequency of hemicraniectomy in patients with acute ischemic stroke. Clin Neuroradiol. 2017;27(2):193-197. doi: 10.1007/s00062-015-0478-8 [DOI] [PubMed] [Google Scholar]
- 34.Rumalla K, Ottenhausen M, Kan P, Burkhardt JK. Recent nationwide impact of mechanical thrombectomy on decompressive hemicraniectomy for acute ischemic stroke. Stroke. 2019;50(8):2133-2139. doi: 10.1161/strokeaha.119.025063 [DOI] [PubMed] [Google Scholar]
- 35.Göttsche J, Flottmann F, Jank L, et al. Decompressive craniectomy in malignant MCA infarction in times of mechanical thrombectomy. Acta Neurochir. 2020;162(12):3147-3152. doi: 10.1007/s00701-019-04180-0 [DOI] [PubMed] [Google Scholar]
- 36.Zaidat OO, Liebeskind DS, Jadhav AP, et al. Impact of age and Alberta Stroke Program Early Computed Tomography score 0 to 5 on mechanical thrombectomy outcomes: analysis from the STRATIS registry. Stroke. 2021;52(7):2220-2228. doi: 10.1161/strokeaha.120.032430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng FC, Yassi N, Sharma G, et al. Cerebral edema in patients with large hemispheric infarct undergoing reperfusion treatment: a HERMES meta-analysis. Stroke. 2021;52(11):3450-3458. doi: 10.1161/strokeaha.120.033246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaidat OO, Kasab SA, Sheth S, et al. TESLA Trial: Rationale, Protocol, and Design. Stroke: Vascular and Interventional Neurology 2023;0:e000787. [Google Scholar]
- 39.Campbell BCV, Hill MD, Nguyen TN, Broderick JP. Acute and interventional treatments. Stroke. 2023;54(2):591-594. doi: 10.1161/strokeaha.122.041254 [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Zhang R, Zhou Y, et al. Patients with ischemic core ≥70 ml within 6 h of symptom onset may still benefit from endovascular treatment. Front Neurol. 2018;9:933. doi: 10.3389/fneur.2018.00933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaesmacher J, Chaloulos-Iakovidis P, Panos L, et al. Mechanical thrombectomy in ischemic stroke patients with Alberta Stroke Program Early Computed Tomography score 0-5. Stroke. 2019;50(4):880-888. doi: 10.1161/STROKEAHA.118.023465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kakita H, Yoshimura S, Uchida K, et al. Impact of endovascular therapy in patients with large ischemic core: subanalysis of recovery by endovascular salvage for cerebral ultra-acute embolism Japan registry 2. Stroke. 2019;50(4):901-908. doi: 10.1161/strokeaha.118.024646 [DOI] [PubMed] [Google Scholar]
- 43.Kerleroux B, Janot K, Dargazanli C, et al. Perfusion imaging to select patients with large ischemic core for mechanical thrombectomy. J Stroke Cerebrovasc Dis. 2020;22(2):225-233. doi: 10.5853/jos.2019.02908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarraj A, Hassan AE, Savitz S, et al. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient's selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol. 2019;76(10):1147-1156. doi: 10.1001/jamaneurol.2019.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang S, Peng Y, Jing CH, et al. Endovascular thrombectomy can be beneficial to acute ischemic stroke patients with large infarcts. J Neurosurg. 2018:1-8. doi: 10.3171/2017.11.JNS171297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations may be shared at the request of any qualified investigator for purposes of replicating procedures and results.