Abstract
Imaging studies of treatment-resistant depression (TRD) have examined brain activity, structure, and metabolite concentrations to identify critical areas of investigation in TRD as well as potential targets for treatment interventions. This chapter provides an overview of the main findings of studies using three imaging modalities: structural magnetic resonance imaging (MRI), functional MRI (fMRI), and magnetic resonance spectroscopy (MRS). Decreased connectivity and metabolite concentrations in frontal brain areas appear to characterize TRD, although results are not consistent across studies. Treatment interventions, including rapid-acting antidepressants and transcranial magnetic stimulation (TMS), have shown some efficacy in reversing these changes while alleviating depressive symptoms. However, comparatively few TRD imaging studies have been conducted, and these studies often have relatively small sample sizes or employ different methods to examine a variety of brain areas, making it difficult to draw firm conclusions from imaging studies about the pathophysiology of TRD. Larger studies with more unified hypotheses, as well as data sharing, could help TRD research and spur better characterization of the illness, providing critical new targets for treatment intervention.
Keywords: Functional MRI, Connectivity, Magnetic resonance spectroscopy, Structural MRI, Ketamine, Psilocybin, Transcranial magnetic stimulation, Electroconvulsive therapy
1. Introduction
Treatment-resistant depression (TRD) is broadly defined as lack of remission in response to at least one antidepressant pharmacological treatment trial that was administered at an adequate dose for an adequate length of time (Ionescu et al., 2015). Given that approximately one-third of individuals diagnosed with depression fail to respond to treatment, one priority in depression research involves using neuroimaging techniques to help identify brain areas that serve as markers for diagnosing TRD or determining potential treatment strategies. This chapter explores imaging research in TRD, with a focus on functional magnetic resonance imaging (fMRI), structural MRI (MRI), and magnetic resonance spectroscopy (MRS). From the outset, it is worth noting that the aforementioned definition of TRD is one of several currently in use. Certain research studies employ very stringent criteria to classify patients with depression as treatment-resistant. However, because a limited number of such studies exist, this chapter used a more lenient set of criteria to select imaging studies of TRD. The studies are summarized below, with an emphasis on highlighting brain areas most likely to reflect altered activity, structure, and metabolite concentrations in TRD as well as imaging evidence describing which interventions—pharmacological or not—may reverse or improve these changes. The insights and challenges of TRD imaging research are also discussed.
2. Functional magnetic resonance imaging and TRD
Functional magnetic resonance imaging (fMRI) is a non-invasive neuroimaging technology that measures and maps brain activity based on changes in blood oxygenation. When neurons are active, they require more oxygen, resulting in locally increased cerebral blood flow to those regions. The slight concentration and signal difference between oxygenated (diamagnetic) and de-oxygenated (paramagnetic) hemoglobin leads to the blood oxygen level dependent (BOLD) contrast. The BOLD signal enables researchers to identify, with high spatial resolution, intrinsic brain fluctuations in regions that are active at rest (resting-state fMRI or rs-fMRI) or while performing a specific task. Both task-based and rs-fMRI studies have provided key insights into the pathophysiology of depression in general and TRD in particular. For example, the “triple network model”, which suggests that depression is due to abnormalities in the salience network (SN), central executive network (CEN), and default mode network (DMN) partly relied on findings obtained via rs-fMRI (Menon, 2011). In addition, several task-based and rs-fMRI studies comparing individuals with TRD to healthy volunteers (HVs) before and after particular interventions found that abnormal functional connectivity in the DMN may be particularly important in TRD (Lener and Iosifescu, 2015; Runia et al., 2022).
The fMRI studies that compared individuals with TRD to treatment responders as well as to HVs are discussed below. Baseline differences that underlie TRD depression are discussed in the context of how brain activity and connectivity in TRD change in response to treatment. Those brain areas whose baseline activity and connectivity could predict treatment response are also highlighted; these studies are summarized in Table 1 and Fig. 1.
Table 1.
fMRI studies in TRD
| Article | Imaging modality | Aims | Experimental design | Summary of findings |
|---|---|---|---|---|
| Kumari et al. (2003) | Tasked-based fMRI Passive viewing of positive, negative, and neutral picture-caption pairs | To compare neural responses to mood-inducing stimuli between patients with TRD and healthy HVs. | TRD group: N = 6 (6 female, mean age = 47) HV group: N = 6 (6 female, mean age = 44) |
TRD participants had decreased activity in the ACC in response to positive and negative picture-caption pairs and increased activity in the right inferior and left middle temporal gyri in response to negative picture-caption pairs. In response to positive picture-caption pairs, decreased activity was observed in the medial frontal gyrus and left hippocampus, and increased activity was observed in the right parahippocampal gyrus, left inferior frontal gyrus, right subgenual anterior cingulate, right striatum, and left brainstem in patients with TRD. |
| Lui et al. (2011) | rs-fMRI. ROI analysis | To compare brain networks in patients with TRD, non-treatment refractory depression, and HVs using rs-fMRI. | TRD group: N = 28 (10 female, mean age = 33) Non-refractory group: N = 32 (11 female, mean age = 32) HV group: N = 48 (17 female, mean age = 35) |
The TRD and non-refractory groups exhibited decreased connectivity in prefrontal-limbic-thalamic areas bilaterally compared to HVs. Compared to the non-refractory group, the TRD group showed decreased connectivity in the left amygdala-ACC-right insula-precuneus. |
| Wu et al. (2011) | rs-fMRI. Regional homogeneity analysis | To identify imaging markers that distinguish TRD, non-treatment refractory depression, and HVs using rs-fMRI and regional homogeneity analyses. | TRD group: N = 22 (7 female, mean age = 35) Non-refractory group: N = 22 (12 female, mean age = 35) HV group: N = 26 (10 female, mean age = 33) |
Compared to HVs, the non-refractory and TRD groups exhibited higher synchronization in temporal and limbic brain areas and lower synchronization in frontal and parietal regions and the caudate. Compared to the non-refractory group, the TRD group had higher synchronization in the right middle temporal gyrus, right insula, and middle caudate and lower synchronization in the left precuneus and left inferior frontal gyrus. |
| De Kwaasteniet et al. (2015) | rs-fMRI. ROI functional connectivity analysis | To examine functional connectivity in patients with TRD, non-treatment-refractory depression, and HVs, in three core neurocognitive networks: the SN, CCN, DMN. | TRD group: N = 17 (9 female, mean age = 52.5) Non-refractory group: N = 18 (12 female, mean age = 48.9) HV group: N = 18 (10 female, mean age = 51.5) |
The TRD group exhibited decreased connectivity between the CCN and DMN as well as between the posterior and anterior DMN compared to the non-treatment refractory and HV groups. |
| Murrough et al. (2015) | Task-based fMRI viewing and rating the emotional valence of positive, negative, and neutral faces | To examine changes in neural activity during positive and negative emotion perception after ketamine administration in patients with TRD. | TRD group: N = 18 (8 female, mean age = 38.1) HV group: N = 20 (9 female, mean age = 35) Intervention: one ketamine infusion (0.5mg/kg) |
Compared to HVs, the TRD group demonstrated decreased neural response to positive faces in the right caudate and insula at baseline. After ketamine, the TRD group showed increased activity in the right caudate in response to positive faces. |
| Carhart-Harris et al. (2017) | rs-fMRI | To examine changes in brain function before versus after psilocybin in patients with TRD. | TRD group: N = 15 (4 female, mean age = 42.8) Intervention: Two doses of psilocybin (10mg and 25mg), given 1 week apart | Patients with TRD exhibited increased resting-state functional connectivity within the DMN following psilocybin treatment. |
| Ferri et al. (2017) | Task-based fMRI. Participants indicated the emotion, gender, or passively observed positive and negative faces | To examine amygdala reactivity and its association with depressive symptoms and treatment response in patients with TRD. | TRD group: N = 80 (58 female, mean age = 41.81) HV group: N = 37 (27 female, mean age = 42.57 |
Compared to HVs, patients with TRD exhibited blunted amygdala activity during affect recognition, which was linked to increased depression severity. |
| Ge et al. (2017) | rs-fMRI | To characterize brain dysfunction in patients with TRD who received rTMS to identify neuroimaging markers that distinguish rTMS responders from non-responders. | TRD group: N = 18 (11 female, mean age = 43) HV group: N = 21 (14 female, mean age = 36.48) Intervention: 20 sessions of rTMS |
Patients with TRD who responded to rTMS exhibited higher functional connectivity within the DMN and SN at baseline compared to non-responders. Functional connectivity of the DMN and SN at baseline predicted clinical improvement in responders. |
| Evans et al. (2018b) | rs-fMRI. ROI analysis | To examine the effect of one ketamine infusion on resting-state DMN two and 10 days post-infusion in patients with TRD and HVs. | TRD group: N = 33 (20 female, mean age = 36) HV group: N = 25 (15 female, mean age = 33) Intervention: one IV ketamine infusion (0.5 mg/kg) |
At baseline, the TRD group had reduced connectivity within the DMN compared to the HVs in the right DLPFC and left postcentral gyrus. Connectivity with the DMN and insula increased 2 days post-infusion for the TRD group, becoming similar to that of HVs, but returned to baseline levels 10 days post-infusion. |
| Roseman et al. (2018) | Task-based fMRI. Passive viewing of positive, negative, and neutral faces | To examine the antidepressant effect of psilocybin on amygdala responses to emotional information among patients with TRD using fMRI. | TRD group: N = 19 (6 female, mean age = 44.7) Intervention: Two doses of psilocybin (10mg and 25mg) |
Patients with TRD exhibited increased activity in the right amygdala in response to emotional faces 1 day post-psilocybin. This response to fearful faces was linked with clinical improvement 1 week later. |
| Chen et al. (2019) | rs-fMRI. ROI analysis | To determine the effects of ketamine on the functional connectivity of networks in the prefrontal cortex in patients with TRD. | TRD group: N = 48 (35 female, mean age = 45.9) Intervention: One infusion of IV ketamine (0.2 mg/kg (low dose) or 0.5mg/kg (high dose)) or placebo |
For both ketamine doses, patients with TRD exhibited reduced connectivity of the left dorsal ACC and DLPFC with frontal and parietal areas post-treatment. The low dose group had increased connectivity of the right dorsal ACC and right DLPFC with the right anterior middle temporal gyrus and left superior parietal lobe, respectively. |
| Luan et al. (2019) | rs-fMRI | To investigate the functional connectivity of the habenular nucleus in patients with TRD. | TRD group: N = 15 (6 female, mean age = 34.43) HV group: N = 15, (8 female, mean age = 33.5) |
Patients with TRD exhibited increased connectivity in the medial superior frontal gyrus, ACC, and medial orbitofrontal gyrus and decreased connectivity in the corpus callosum with the right habenular nucleus. Increased connectivity was observed in the inferior temporal gyrus and decreased connectivity was observed between the insula and the left habenular nucleus. Patients with TRD exhibited abnormal connectivity between the habenula and DMN. |
| Ge et al. (2020) | rs-fMRI | To examine whether the connectivity of the subgenual and rostral ACC can predict treatment response to rTMS. | TRD group: N = 50 (29 female, mean age = 43.7) HV group: N = 24 (12 female, mean age = 45.25) Intervention: 20–30 sessions of rTMS over the left DLPFC |
At baseline, increased connectivity between the right ACC and left lateral parietal cortex as well as decreased connectivity between the sgACC and right DLPFC in patients with TRD predicted treatment response to rTMS. Following treatment, the hyperconnectivity of the sgACC and visual cortex in patients with TRD decreased to levels comparable to HVs. |
| Loureiro et al. (2020) | Task-based fMRI. Face (happy, neutral, or fearful) and object matching paradigm. | To investigate the neural effects of ketamine and ECT on emotion processing in patients with TRD. | TRD group: N = 44 (23 female, mean age = 37.1) HV group: N = 32 (19 female, mean age = 34.45) Intervention: Four IV ketamine infusions (0.5 mg/kg) or ECT (until maximal response, mean = 14 sessions) |
Both interventions reduced amygdala activity in response to happy and fearful faces and increased activity in the DLPFC and insula in response to fearful faces in patients with TRD. |
| Mertens et al. (2020) | Task-based fMRI. Passive viewing of positive, negative, and neutral faces | To investigate the neural mechanisms underlying changes in amygdala reactivity after psilocybin in patients with TRD. | TRD group: N = 19 (6 female, mean age = 44.7) Intervention: Two doses of psilocybin (10mg and 25mg) | In response to fearful faces, patients with TRD exhibited decreased connectivity between the right amygdala and vmPFC after psilocybin. Increased connectivity between the amygdala and vmPFC to occipital-parietal cortices during emotion processing was also observed post-psilocybin. |
| Sahib et al. (2022) | rs-fMRI | To investigate resting-state functional connectivity of the brain after acute and serial ketamine administration in patients with TRD. | TRD group: N = 61 (29 female, mean age = 38.96) HV group: N = 60 (24 female, mean age = 32.87) Intervention: Four IV ketamine infusions (0.5mg/kg) |
Compared to HVs, patients with TRD exhibited increased connectivity between the DMN and visual cortex at baseline. This hyperconnectivity decreased after ketamine treatment, trending towards that of HVs. |
| Sahib et al. (2020) | Task-based fMRI. Go/NoGo paradigm | To examine ketamine’s effects on the inhibitory control network in patients with TRD. | TRD group: N = 47 (19 female, mean age = 38.61) HV group: N = 32 (18 female, mean age = 34.75) Intervention: Four IV ketamine infusions (0.5mg/kg) |
Following serial ketamine infusions, patients with TRD exhibited reduced activity in the inhibitory control network, DMN, and SN. Lower activity in the SMA at baseline was linked with remission, with ketamine normalizing SMA activity towards that of HVs. |
| Loureiro et al. (2021) | Task-based fMRI. Go/NoGo paradigm | To investigate ketamine’s effects on cerebro-cerebellar circuitry during response-inhibition in patients with TRD. | TRD group: N = 46 (18 female, mean age = 39.2) HV group: N = 32 (18 female, mean age = 34.75) Intervention: Four IV ketamine infusions (0.5mg/kg) |
Patients with TRD who achieved remission had higher connectivity of the cerebellum with the SN and FPN compared to nonremitters and HVs. Decreased connectivity of the cerebellum with the SMN and FPN post-ketamine was observed among remitters only. |
| Nakamura et al. (2021) | rs-fMRI. Seed-based analysis | To examine whether rs-fMRI can reveal predictors of ketamine response in patients with TRD. | TRD group: N = 15 (9 female, mean age = 45.9) Intervention: Four IV ketamine infusions (0.5 mg/kg) |
Decreased connectivity between the right amygdala and sgACC at baseline predicted response to ketamine in patients with TRD. |
| Rivas-Grajales et al. (2021) | rs-fMRI Seed-voxel functional connectivity analysis | To examine functional connectivity changes of the habenula after a single dose of ketamine in patients with TRD. | TRD group: N = 35 (16 female, mean age = 42.2) Intervention: One IV ketamine infusion (0.5 mg/kg) |
Increased connectivity between the right habenula and a cluster in the right frontal pole post-ketamine was associated with reduced depressive symptoms in patients with TRD. Reduced severity of depressive symptoms was also linked to increased connectivity between the right habenula and the right occipital pole, right temporal pole, right parahippocampal gyrus, and left lateral occipital cortex. |
| Siegel et al. (2021) | rs-fMRI | To examine neurobiological mechanisms underlying ketamine’s antidepressant effects using rs-fMRI in patients with TRD. | TRD group: N = 23 (10 female, mean age = 40) HV group: N = 27 (17 female, mean age = 34.2) Intervention: One 96-hour IV ketamine infusion (0.6 mg/kg/h) |
At baseline, patients with TRD exhibited increased connectivity in the DMN and limbic system and between the sgACC and DMN. After ketamine, this hyperconnectivity decreased and became comparable to that of HVs. |
| Vasavada et al. (2021) | rs-fMRI | To investigate connectivity changes between limbic structures and resting-state networks following ketamine treatment in patients with TRD. | TRD group: N = 44 (18 female, mean age = 38.2) HV group: N = 50 (27 female, mean age = 32.3) Intervention: Four IV ketamine infusions (0.5 mg/kg) |
At baseline, patients with TRD had decreased connectivity between the right amygdala and the CEN compared to HVs. After ketamine, connectivity between the right amygdala and right CEN and between the left amygdala and SN decreased, showing normalization to connectivity observed in HVs and suggesting restoration of top-down emotional processing in patients with TRD. |
| Godfrey et al. (2022) | rs-fMRI | To investigate how functional connectivity predicts response to, and changes after, rTMS in patients with TRD. | TRD group: N = 26 (10 female, mean age = 41.2) Intervention: 20 rTMS sessions |
After rTMS, patients with TRD had reduced connectivity in the SN, particularly in the prefrontal cortex, and this reduction was linked to clinical response to rTMS. |
| Sun et al. (2023) | rs-fMRI | To compare functional connectivity in the DMN, SN, CCN, and AN between patients with TRD, non-treatment refractory patients, and HVs. | TRD group: N = 38 (22 female, mean age = 42.21) Non-TRD group: N = 39 (24 female, mean age = 41.6) HV group: N = 40 (23 female, mean age = 40.9) |
Compared to HVs, participants with TRD and non-TRD exhibited hyperconnectivity of the DMN and abnormal connectivity in the AN, SN, and CCN. Compared to HVs and participants with non-TRD, patients with TRD exhibited increased connectivity in the SN. |
ACC: anterior cingulate cortex; AN: affective network; CCN: cognitive control network; CEN: central executive network; DLPFC: dorsolateral prefrontal cortex; DMN: default mode network; ECT: electroconvulsive therapy; FPN: frontoparietal network; HV: healthy volunteer; IV: intravenous; rs-fMRI: resting-state functional magnetic resonance imaging; rTMS: repetitive transcranial magnetic stimulation; sgACC: subgenual anterior cingulate cortex; SMA: somatosensory cortex; SN: salience network; TRD: treatment-resistant depression; vmPFC: ventral medial prefrontal cortex.
FIG. 1.
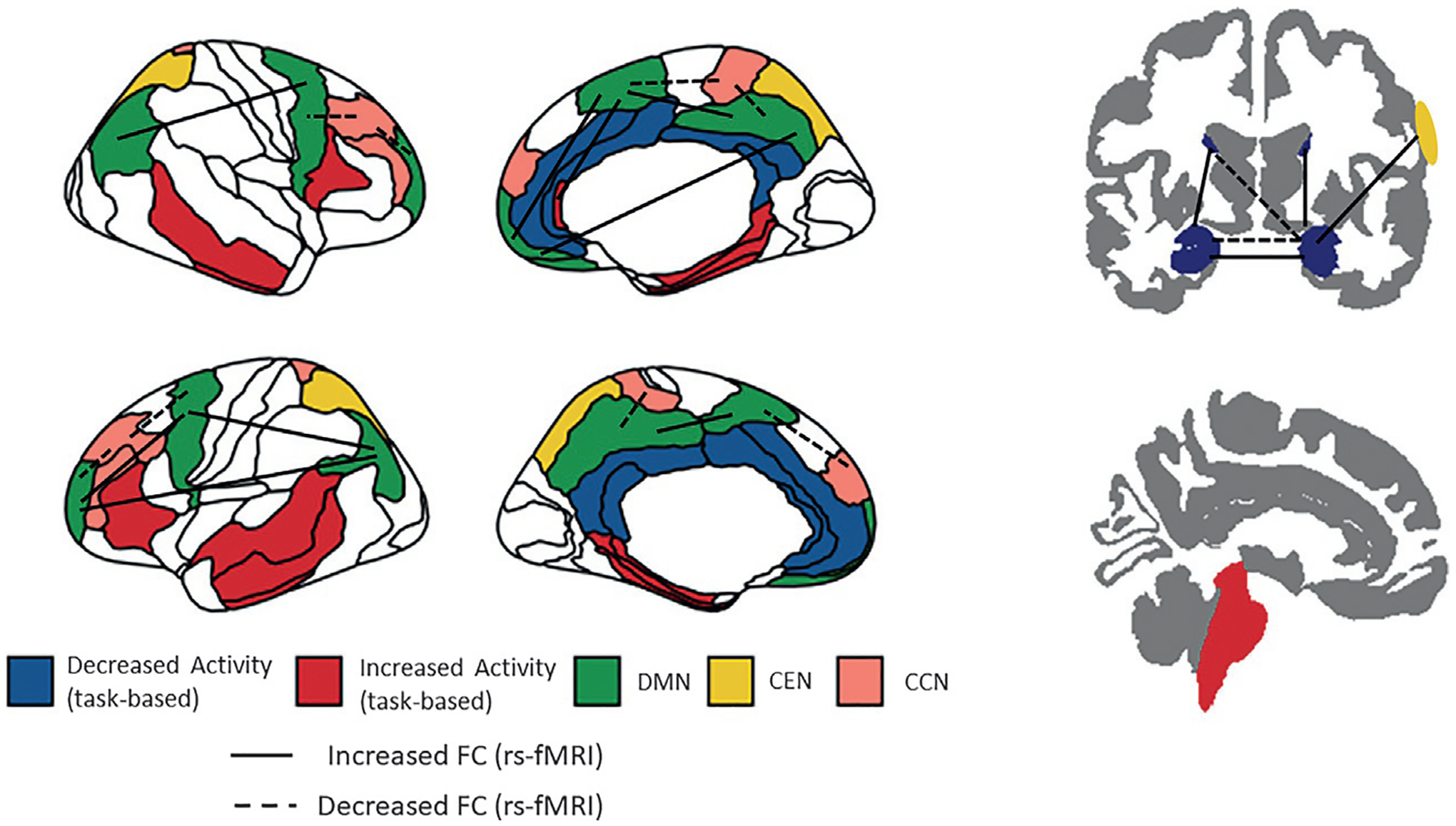
Schematic representation of the increases and decreases in connectivity and brain activations in treatment-resistant depression (TRD). The increases and decreases in connectivity between brain networks and brain areas are identified in comparison to healthy volunteers (HVs).
3. Brain connectivity and activity in TRD
3.1. Evidence from rs-fMRI studies
Regional rs-fMRI brain connectivity studies in individuals with TRD use brain regions of interest (ROIs) important to emotional (amygdala, thalamus, hippocampus) and reward (striatal areas) processing and which are known to be implicated in the pathophysiology of depression. One study found increased amygdalar and hippocampal connectivity to the SN and CEN between the right amygdala and right CEN for individuals with TRD compared to HVs (Vasavada et al., 2021). Another study examined connectivity within the limbic system—including the amygdala, hippocampus, and nucleus accumbens as well as connectivity of the subgenual anterior cingulate cortex (sgACC) with the rest of the brain—and found increased connectivity between regions of the limbic system and between the sgACC and the DMN in individuals with TRD compared to HVs (Siegel et al., 2021). The same study also found decreased connectivity between the right intermediate hippocampus and limbic areas in individuals with TRD that correlated with the duration of the depressive episode. Another study that examined connectivity between the habenula and the rest of the brain identified increased connectivity between the right habenular nucleus and the medial superior frontal gyrus as well as between the anterior cingulate cortex (ACC) and medial orbitofrontal gyrus (Luan et al., 2019). Decreased connectivity with the corpus callosum was also observed in individuals with TRD compared to HVs. For the left habenular seed, increased functional connectivity between this seed and the inferior temporal gyrus and decreased functional connectivity with the insula was also observed in individuals with TRD (Luan et al., 2019).
Network connectivity studies in TRD have mainly focused on the DMN and SN, given that depression is associated with DMN hyperconnectivity (Menon, 2011) and reward processing deficits are linked to anhedonia (Kelly et al., 2022). Hyperconnectivity within the DMN was identified in two studies comparing individuals with TRD to HVs (Siegel et al., 2021; Sun et al., 2023). In contrast, Evans and colleagues reported decreased connectivity of the DMN with the dorsal lateral prefrontal cortex (DLPFC) and the left post-central gyrus in individuals with TRD (Evans et al., 2018b). Increased connectivity within the SN has also been identified in individuals with TRD (Sun et al., 2023).
3.2. Evidence from task-based fMRI
Emotional and reward processing tasks as well as tasks that engage attentional and inhibitory processes have been used to examine the changes in brain activity that underlie TRD. The main target area of the emotional processing tasks is the amygdala, as deficits in emotional processing in depression have been linked to hyperactivation of that brain area, especially for negative information. Blunted amygdala activity in response to emotional processing has been identified and linked to greater severity of depressive symptoms (Ferri et al., 2017), although another study found no differences in the amygdala activity of individuals with TRD versus HVs during the processing of sad and happy faces (Loureiro et al., 2020). When the activation of other brain areas was examined, individuals with TRD showed reduced neural responses to positive faces within the right caudate (Murrough et al., 2015). During the processing of pairs of positive and negative pictures and captions, a relative decrease in ACC activation during the processing of both negative and positive pictures was noted for individuals with TRD, as well as a decrease in the medial frontal gyrus and left hippocampus with positive picture-caption pairs. Increased responses were also observed in individuals with TRD in the right inferior and left middle temporal gyri with negative picture-caption pairs, and in the right parahippocampal gyrus, left inferior frontal gyrus, right sgACC, right striatum, and left brain stem with positive picture-caption pairs (Kumari et al., 2003).
Inhibition control has been examined using the Go/NoGo task, which requires participants to withhold responses to certain items in a series of stimuli. The task engages brain areas associated with cognitive control, including frontal and parietal regions as well as the somatosensory cortex (SMA), the striatum, the thalamus, and the cerebellum (Simmonds et al., 2008). In this task, no differences in brain activity were seen between individuals with TRD and HVs (Sahib et al., 2020).
3.3. TRD compared to major depressive disorder (MDD)
To better understand TRD, it is necessary to examine the changes in brain activity and connectivity that distinguish individuals with TRD not only from HVs but also from individuals with major depressive disorder (MDD) who responded to treatment. In one study that examined the synchronization of fMRI signal oscillations using an rs-fMRI connectivity approach, both TRD and non-TRD participants showed higher synchronization in temporal and limbic brain areas compared to HVs; the opposite pattern was observed in frontal and parietal regions as well as the caudate. When compared to treatment responders, individuals with TRD showed higher synchronization in the right middle temporal gyrus, right insula, and middle cingulate, and lower synchronization in the left precuneus and left inferior frontal gyrus (Wu et al., 2011). Another study examined differences between the SN, the cognitive control network (CCN), and the DMN and found that, compared to HVs and individuals with MDD who responded to treatment, individuals with TRD showed decreased connectivity between the CCN and DMN and reduced connectivity between the anterior and posterior DMN (De Kwaasteniet et al., 2015). Finally, another study that examined brain connectivity between 13 ROIs found significantly reduced connectivity within prefrontal-limbic-thalamic areas bilaterally when individuals with TRD and treatment responders with MDD were compared to HVs. Direct comparison of individuals with TRD and treatment responders with MDD revealed decreased connectivity within the left amygdala-ACC-right insula-precuneus region for those with TRD (Lui et al., 2011).
3.4. TRD brain connectivity and activity in response to interventions
Rapid-acting antidepressants, including ketamine and psilocybin, have shown great promise in alleviating depressive symptoms in individuals with TRD (Bratsos and Saleh, 2019). Evidence from clinical and preclinical studies suggests that these compounds exert their antidepressant action by inducing changes in brain plasticity (Zanos and Gould, 2018). However, the effects of these compounds on brain activity and connectivity remain largely unexplored. In addition, identifying brain areas that predict treatment response to these compounds would enable patient stratification and a more effective and personalized approach for the treatment of TRD.
3.5. Ketamine—Evidence from rs-fMRI studies
The effects of two doses of ketamine (0.2 and 0.5 mg/kg) were compared to placebo in an rs-fMRI study focusing on connectivity of the prefrontal cortex (PFC), mainly the ACC and DLPFC. Twenty-four hours post-infusion, both ketamine doses reduced connectivity of the left dorsal ACC and DLPFC with frontal and parietal brain areas. For the lower dose, increased connectivity was also observed between the DLPFC and the left superior parietal lobe and between the right ACC and the right anterior middle temporal gyrus (Chen et al., 2019). When habenula connectivity was examined in individuals with TRD at baseline and 24hours after a single ketamine infusion, changes in depressive symptoms, as assessed by two questionnaires—the Montgomery-Asberg Depression Rating Scale (MADRS) and the Quick Inventory of Depressive Symptomatology (Self-Report) (QIDS-SR)—were associated with increased connectivity between the right habenula and a cluster in the right frontal pole and between the right habenula and clusters in the right occipital pole, right temporal pole, right parahippocampal gyrus, and left lateral occipital cortex, respectively (Rivas-Grajales et al., 2021).
These studies, though limited in number and lacking replication, are useful for understanding the connectivity changes that accompany ketamine administration. They do not, however, offer any insight into the direction of those changes. Studies comparing HVs and individuals with TRD before and after treatment suggest that ketamine normalizes the altered brain activity and connectivity associated with TRD. For example, Vasavada and colleagues demonstrated that, 24hours after a total of four ketamine infusions administered two to three times a week, connectivity between limbic regions and resting state networks trended towards normalization; ketamine decreased connectivity between the left amygdala and the SN that had appeared to be increased at baseline in individuals with TRD compared to HVs (Vasavada et al., 2021). In the same study, ketamine also increased connectivity between limbic regions and the CEN, an effect that was interpreted as ketamine restoring top-down control of emotional processing, and normalized connectivity of the SMA and ventral attention network. Other studies found that ketamine increased fronto-striatal connectivity (Mkrtchian et al., 2021) and decreased DMN connectivity with the visual cortex (Sahib et al., 2022), sgACC (Siegel et al., 2021), and insula (Evans et al., 2018b) to levels similar to those of HVs. However, when connectivity was examined approximately 10 days after a single ketamine infusion (Evans et al., 2018b; Mkrtchian et al., 2021), the effects of the drug had already dissipated, indicating that these changes were neither permanent nor long-lived, and that multiple infusions might be required to sustain ketamine’s antidepressant effects. With regard to predicting treatment response, decreased connectivity between the left amygdala and the SN at baseline predicted improvement in anxiety symptoms 24hours and 4 weeks post-ketamine, while connectivity of the left hippocampus with the CEN predicted decreased anhedonia post-ketamine (Vasavada et al., 2021). In another study, connectivity between the amygdala and the sgACC at baseline predicted treatment response to ketamine in individuals with TRD (Nakamura et al., 2021).
3.6. Ketamine—Evidence from task-based fMRI studies
In emotional processing tasks 24hours post-administration, ketamine increased response in the right caudate during the processing of positive faces in individuals with TRD (Murrough et al., 2015). When the effects of four ketamine infusions were compared to 14 sessions of electroconvulsive therapy (ECT) in individuals with TRD, both interventions were found to decrease amygdala activity for positive and negative emotional processing; in contrast, increased activity in response to fearful faces was observed in the insula and DLPFC (Loureiro et al., 2020). These findings suggest that the two interventions share common brain targets through which they may exert antidepressant effects. Another study of ketamine’s effects on response inhibition (the Go/NoGo task) found that ketamine decreased brain activity in the inhibitory control network, as well as in large scale brain networks (including the DMN and the SN) 24hours post-administration (Sahib et al., 2022). In that study, individuals with TRD who achieved remission post-ketamine had lower functional activation in the SMA pre-treatment that normalized post-ketamine. Another study that used the same Go/NoGo task found that individuals with TRD who remitted post-ketamine had decreased connectivity of the cerebellum with frontoparietal regions and the SMA network that normalized after ketamine treatment (Sahib et al., 2022).
3.7. Psilocybin—Evidence from fMRI studies
In individuals with TRD, psilocybin was found to increase functional connectivity in the DMN post-treatment (Carhart-Harris et al., 2017). Psilocybin also increased response to fearful faces (Roseman et al., 2018) in the right amygdala and decreased connectivity between the right amygdala and the ventral medial prefrontal cortex (vmPFC) during emotional processing (Mertens et al., 2020). The same studies found increased resting-state connectivity between the vmPFC and the bilateral inferior lateral cortex (Carhart-Harris et al., 2017; Mertens et al., 2020); in addition, decreased connectivity between the parahippocampal regions and the PFC predicted treatment response (Carhart-Harris et al., 2017), while increased amygdala response, at baseline and in relation to fearful faces, predicted improvement in depressive symptoms (Roseman et al., 2018).
3.8. Other non-pharmacological interventions
TMS, which involves magnetic focal stimulation of brain areas critical to mood regulation and depression, is mainly executed in the DLPFC (Gaynes et al., 2014). One study that compared individuals with TRD to HVs found that, in those with TRD scanned at baseline and 12 weeks after TMS, treatment response was associated with lower functional connectivity of the sgACC with the right DLPFC and higher connectivity of the right ACC with the left lateral parietal cortex measured at baseline (Ge et al., 2020). The same study found that hyperconnectivity between the sgACC visual cortex normalized to a level comparable to that of HVs. When individuals who responded to TMS were compared to those who did not, TMS responders were found to have higher functional connectivity within the DMN and the SN (Ge et al., 2017). Finally, another study of individuals with TRD found that clinical response to TMS correlated with reduced functional connectivity from baseline to post-TMS within the SN (Godfrey et al., 2022).
4. Structural MRI changes in TRD
Structural MRI allows both the anatomy and pathology of the brain to be examined. Analyses, including voxel-based morphometry (VBM), can be applied to process structural MRI images and quantify the amount of grey matter, white matter, and cerebrospinal fluid (CSF) contained in each brain voxel, yielding measures of total brain volume and grey and white matter integrity in specific brain areas. White matter integrity can also be assessed using diffusion tensor imaging (DTI), where the intensity of the MR signal is based on water diffusivity. Following the direction of water molecules, which tend to diffuse parallel to nerve fibers due to the presence of myelin, DTI allows white matter tracts in the brain to be assessed. Different DTI measures are used to assess different aspects of white matter integrity, including fractional anisotropy (FA), which gives information about the integrity of tissue microstructure, mean diffusivity (MD), which assesses white matter density, and mode of anisotropy, which provides information about crossing fibers.
Structural MRI studies examining the grey and white matter brain changes associated with depression found that MDD is characterized by decreased brain volume in the ACC and have highlighted the importance of the hippocampus and amygdala as brain areas where structural changes could be linked to depressive symptoms (for a review, see Scheepens et al., 2020). Greater structural brain changes also seem to be directly associated with duration of illness (Lorenzetti et al., 2009). This could be particularly interesting with regard to TRD, as the refractory nature of the illness could lead to comparatively longer-lasting depressive episodes. In that case, greater structural brain changes could be at least partly responsible for the greater cognitive deterioration that usually characterizes individuals with TRD (Rao et al., 2019).
The sections that follow summarize the key structural MRI findings in TRD. Changes in brain volume and white matter integrity are reviewed in an effort to identify brain areas whose structure is altered in TRD and how these areas could be linked to TRD symptomatology.
4.1. Brain volume and grey matter integrity in TRD
Studies exploring structural changes in individuals with TRD versus HVs have mainly focused on the frontal and medial temporal cortical areas as well as the ACC and hippocampus (for a review, see Klok et al., 2019). Specifically, reduced brain volume was identified in the ACC and the adjacent area of the corpus callosum (CC) (Zhang et al., 2009) as well as in a cluster that included the medial frontal gyrus, the ACC, and the medial orbital frontal cortex (Lan et al., 2016). Significant reductions in brain volume were also identified in individuals with TRD in the right superior frontal gyrus, the left medial frontal gyrus, and the left cingulate gyrus (Serra-Blasco et al., 2013). When the brain volume of the ACC was examined in more detail, both the dorsal and ventral components of the ACC had decreased brain volume in individuals with TRD (Machino et al., 2014). The structural integrity of the hippocampus has also been investigated in individuals with TRD compared to HVs, with studies reporting reduced volume in the left hippocampus (Mervaala et al., 2000; Shah et al., 1998) and the hippocampal tail (Maller et al., 2012) as well as bilaterally (Young, 2013). Other brain areas associated with reduced brain volume in TRD include the amygdala and the insula (Lan et al., 2016; Young, 2013), striatal areas including the caudate (Ma et al., 2012; Shah et al., 2002), medial frontal areas (Serra-Blasco et al., 2013; Shah et al., 2002), and medial temporal areas (Lan et al., 2016; Ma et al., 2012). Interestingly, when gender-based structural differences in the entorhinal cortex were examined, females with TRD had reduced volume compared to female HVs, a finding that did not reach statistical significance when male participants were compared to male HVs (Furtado et al., 2008).
4.2. White matter integrity in TRD
In TRD, white matter integrity studies examined with DTI have focused on white matter tracts that interconnect brain areas that present with altered structure and function. Elevated FA has been observed in the left angular bundle and the right uncinate fasciculus, while elevated MO has been identified in the right uncinate fasciculus (Young, 2013). The angular bundle is a group of white matter tracts that serve as the primary route for neocortical inputs to reach the hippocampus, and the uncinate fasciculus connects the anterior temporal lobe to medial and lateral orbital frontal areas. In contrast to previous findings, reduced FA was found in different white matter tracts that connect neocortical and limbic elements, specifically the limbic lobe uncus and the middle frontal gyrus (Peng et al., 2013) as well as the superior and inferior longitudinal fascicule (De Diego-Adeliño et al., 2014) in individuals with TRD. When compromised, tracts that connect cortical and limbic brain areas could contribute to the cognitive difficulties observed in TRD. When the integrity of the CC—a major bundle of white matter tracts that connects the two hemispheres—was examined, one study found no changes in white matter integrity and volume (Sun et al., 2009), while others reported significant reductions in the body (Guo et al., 2012) and FA (De Diego-Adeliño et al., 2014) of the CC tracts in individuals with TRD. Structural MRI study findings in patients with TRD compared to HVs are schematically presented in Fig. 2.
FIG. 2.
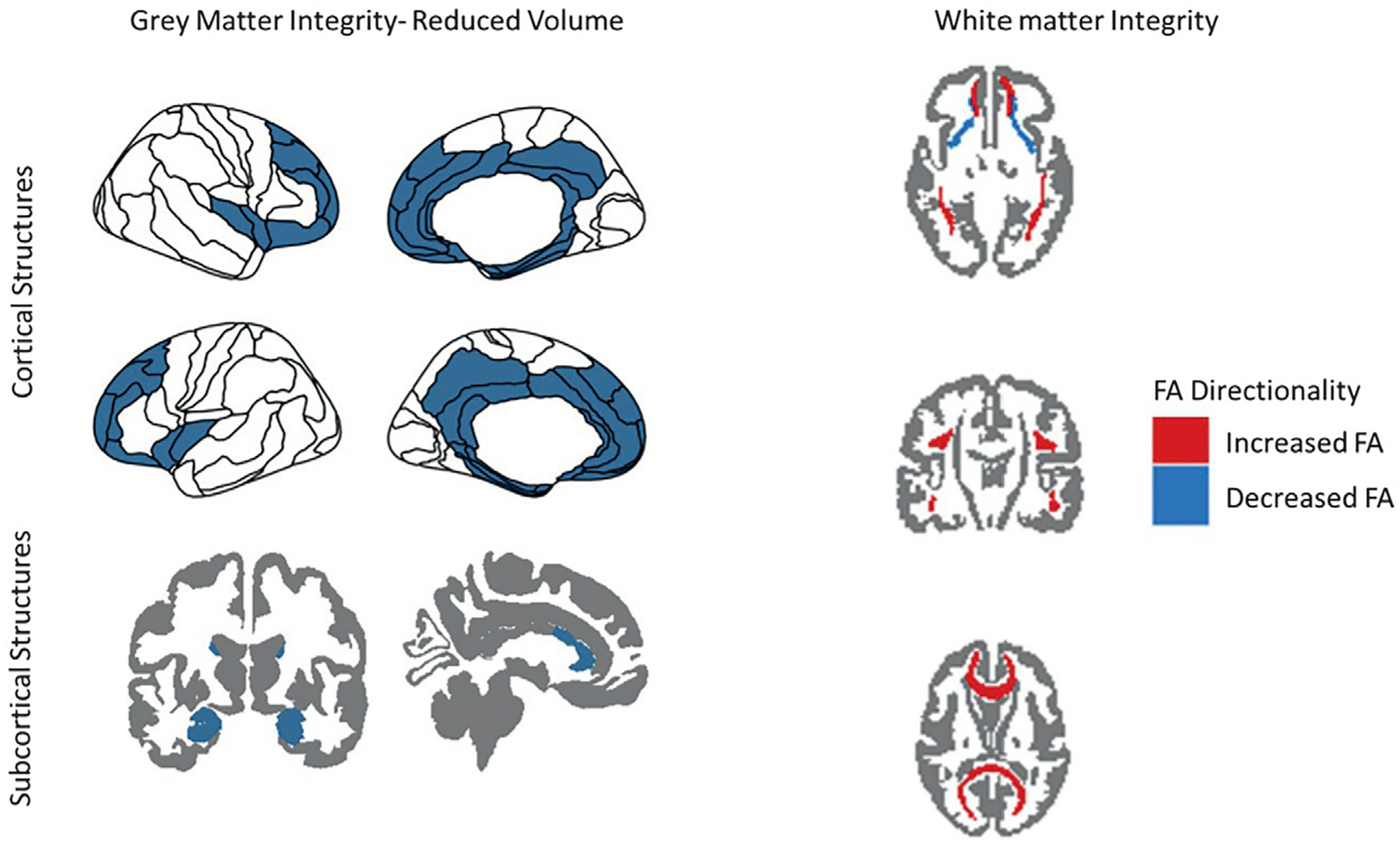
General findings from structural magnetic resonance imaging (MRI) research in treatment-resistant depression (TRD) at baseline. Increases and decreases in brain volume and fractional anisotropy (FA) are with respect to healthy volunteers (HVs).
4.3. The relationship between structural changes, TRD symptoms, illness duration, and treatment interventions
Several researchers have examined whether changes in the structure and white matter integrity of different brain areas are linked to the severity and duration of depressive symptoms. One study found that the brain volume of the bilateral inferior temporal gyrus was negatively correlated with depression scores (Zhang et al., 2009), while another study found that age and illness duration negatively correlated with the reduced FA observed in the left limbic lobe uncus, left middle frontal gyrus, and right cerebellum of individuals with TRD (Serra-Blasco et al., 2013). When specific behaviors associated with depression were examined, rumination was significantly associated with reduced brain volume in the right temporal gyrus (Serra-Blasco et al., 2013). When connectivity of the DMN and the reward processing circuit were examined, brain areas that presented with altered connectivity also presented with reduced brain volume (Ma et al., 2012), which suggests a potential link between structural changes and connectivity changes that could underlie the cognitive and reward processing deficits observed in TRD. Finally, TMS was found to significantly increase the grey matter volume of individuals with TRD in several brain clusters, including the ACC, left middle temporal gyrus, left insula, and right angular gyrus (Lan et al., 2016), suggesting that structural changes might not be irreversible and that their restoration could be linked to symptom improvement. The structural MRI studies on TRD are summarized in Table 2.
Table 2.
Spectroscopy studies in TRD.
| Article | Imaging modality | Aims | Experimental design | Summary of findings |
|---|---|---|---|---|
| Shah et al. (1998) | Voxel-based, whole brain, GM density analysis | To compare cortical GM density between patients with TRD, patients who recovered from depression, and HVs | TRD group: N = 20 (7 female, mean age = 48.9) Recovered group: N = 20 (7 female, mean age = 47.7) HV group: N = 20 (7 female, mean age = 49.3) |
Patients with TRD had reduced GM density in the left temporal cortex, including the left hippocampus. These changes could be associated with the duration of the illness and are linked with the cognitive difficulties that are linked to TRD. |
| Mervaala et al. (2000) | Voxel-based volumetric analysis | To examine amygdala and hippocampal volume in patients with TRD compared to HVs | TRD group: N = 34 (18 female, mean age = 42.2) HV group: N = 17 (11 female, mean age = 42.1) |
Reduced hippocampal volume and amygdala asymmetry (left smaller than right) were observed in the TRD group, highlighting the important role of those two brain areas in depression. |
| Shah et al. (2002) | Voxel-based volumetric analysis | To examine changes in fronto-striatal structures in TRD patients compared to patients who recovered from depression and HVs | TRD group: N = 20 (7 female, mean age = 48.9) Recovered group: N = 20 (7 female, mean age = 47.7) HV group: N = 20 (7 female, mean age = 49.3) |
Patients with TRD exhibited reduced volume in frontal and striatal brain structures. Changes were observed in the PFC as well as temporal structures, the right putamen, and the hippocampus. The degree of these changes was linked to illness duration. |
| Furtado et al. (2008) | Volumetric analysis | To examine possible abnormalities in the volume of the entorhinal cortex | TRD group: N = 45 (23 female, mean age = 37.5) HV group: N = 30 (17 female, mean age = 35.8) |
Compared to HV females, female TRD patients showed a significant reduction in total brain volume as well as volume of the left entorhinal cortex. |
| Sun et al. (2009) | Volumetric analysis | To identify differences in the whole and midsagittal CC in patients with TRD and TRS | TRD group: N = 45 (23 female, mean age = 40.8) TRS group: N = 42 (17 female, mean age = 39.6) HV group: N = 30 (18 female, mean age = 34.6) |
TRS and TRD patients did not significantly differ from HVs in the whole CC area. The CC4 area was significantly reduced in TRD patients compared to HVs. |
| Zhang et al. (2009) | Magnetization transfer imaging and voxel-based morphometry | To identify differences between the individuals with TRD and HVs in the limbic-cortical-striatal-pallidal-thalamic tract | TRD group: N = 15 (5 female, mean age = 33.5) HV group: N = 15 (5 female, mean age = 33.4) |
Reduced magnetization transfer ratio was observed in the TRD group in the ACC, insula, caudate tail, and amygdala-parahippocampal area. No VBM differences were observed between the TRD group and the HVs. |
| Guo et al. (2012) | DTI | To investigate the integrity of white matter tracts of different brain regions in patients with TRD compared to HVs | TRD group: N = 23 (12 female, mean age = 27.4) HV group: N = 19 (9 female, mean age = 24.4) |
Lower FA values were observed in the right anterior limb of the internal capsule, the body of the CC, and the bilateral external capsule in TRD patients compared to HVs. |
| Ma et al. (2012) | VBM and rs-fMRI | To identify GM differences in TRD and patients who responded to treatment and examine whether they are linked with altered functional connectivity | TRD group: N = 18 (7 female, mean age = 27.4) TRS group: N = 17 (7 female, mean age = 26.7) HV group: N = 17 (7 female, mean age = 24.2) |
Reduced GM volume was identified in the right middle temporal gyrus for individuals with TRD and those who had responded to treatment. Reduced GM volume was identified in the caudate of the TRD group. Both patient groups showed altered connectivity between these areas, the DMN, and frontal regions. |
| Maller et al. (2012) | Volumetric analysis | To investigate hippocampal volume changes in individuals with TRD, those with schizophrenia, and HVs | TRD group: N = 182 (88 female, mean age = 42.2) Schizophrenia group: N = 52 (20 female, mean age = 41.0) HV group: N = 76 (35 female, mean age = 35.0) |
Reduced hippocampal volume was identified in the tail section of patients with TRD and schizophrenia. This finding was most pronounced in the schizophrenia group. |
| Peng et al. (2013) | Voxel-based analysis of DTI data | To investigate white matter changes in TRD with specific interest in cortical-limbic, cortical-subcortical, and emotion regulation brain areas | TRD group: N = 30 (11 female, mean age = 26.8) HV group: N = 25 (11 female, mean age = 28.2) |
Decreased FA was identified in the left middle frontal gyrus, left limbic lobe uncus, and right cerebellum posterior lobe of patients with TRD. |
| Serra-Blasco et al. (2013) | VBM | To investigate GM volume in depression | TRD group: N = 22 (18 female, mean age = 49) Remitted, recurrent group: N = 22 (20 female, mean age = 48) First episode depressed group: N = 22 (15 female, mean age = 44) HV group: N = 32 (23 female, mean age = 46) |
In the TRD group, reduced brain volume was observed in frontolimbic areas. This group also showed smaller volumes in the left insula and right medial frontal gyrus compared to the first episode group. The remitted, recurrent group showed increased volume in the bilateral medial frontal gyrus compared to the TRD group. |
| Young (2013) | VBM | To examine differences in fronto-limbic GM volume and the connecting tracts | TRD group: N = 22 (14 female, mean age = 52.1) HV group: N = 21 (no additional information provided) |
Patients with TRD had increased GM volume in the left medial orbitofrontal cortex and bilateral hippocampus. Increased FA was also identified for the left angular bundle and right uncinate fasciculus in patients with TRD. |
| De Diego-Adeliño et al. (2014) | DTI | To investigate whole brain, white matter microstructure abnormalities in TRD | TRD group: N = 18 (15 female, mean age = 48.5) Remitted, recurrent group: N = 15 (14 female, mean age = 47) First episode depressed group: N = 19 (11 female, mean age = 44.2) HV group: N = 17 (12 female, mean age = 43.4) |
Compared to the first episode depressed and HV groups, the TRD group had reduced FA at the cingulum, CC, and superior and inferior longitudinal fascicule. Reduced FA was also observed in the TRD group at the ventromedial PFC compared to the remitted recurrent depressed group. |
| Machino et al. (2014) | VBM | To investigate the relationship between GM volume and rumination | TRD group: N = 29 (13 female, mean age = 39.6) HV group: N = 29 (13 female, mean age = 38.7) |
For the TRD group, decreased GM volume was identified in the left dorsal ACC, right ventral cingulate cortex, right superior frontal gyrus, right cerebellum, and cerebellar vermis. GM volume in the right superior temporal gyrus correlated with rumination. |
| Lan et al. (2016) | VBM | To identify structural brain changes in TRD and how these might change in response to TMS | TRD group: N = 45 (27 female, mean age = 41.5) HV group: N = 42 (27 female, mean age = 39.2) |
Patients with TRD had lower GM volume in several brain areas including the ACC and frontal and temporal cortical areas. TMS increased brain volume in a cluster including the ACC, left medial frontal gyrus and frontal medial orbital gyrus. |
ACC: anterior cingulate cortex; CC: corpus callosum; DMN: default mode network; DTI: diffusion tensor imaging; FA: fractional anisotropy; GM: grey matter; HV: healthy volunteer; PFC: prefrontal cortex; rs-fMRI: resting-state functional magnetic resonance imaging; TRD: treatment-resistant depression; TRS: treatment-resistant schizophrenia; VBM: voxel-based morphometry.
5. MRS and TRD
MRS enables researchers to non-invasively observe biochemical processes in the body by examining metabolite concentrations, based on the fact that atomic nuclei in different molecules experience slightly different magnetic fields due to distinctive electron distributions. The resulting unique signals produce a spectrum with distinct peaks for each metabolite. MRS results are typically quantified as ratios to stable metabolites found in tissue, like creatine.
MRS studies in TRD mainly focus on gamma-aminobutyric acid (GABA) and glutamate levels, which are inhibitory and excitatory neurotransmitters, respectively. GABA and glutamate are also linked biochemically, as GABA is synthesized by glutamate with the action of glutamic acid decarboxylase. Depression has been associated with both GABA-ergic and glutamatergic abnormalities (the “glutamate hypothesis” of depression) with supporting evidence drawn from pharmacological, post-mortem, and genetic studies (Sanacora et al., 2012; Yüksel and Öngür, 2010). These studies indicate that glutamatergic excitatory synaptic transmission appears dysfunctional in depression, especially in brain areas that have been linked to affective and reward processing, which would contribute to the emotional and hedonic processing deficits observed in depression. MRS studies in depression have also explored metabolites such as myo-inositol (m-Ino), N-acetyl aspartate (NAA), choline (Cho), creatinine (Cr) and lactate (Lac), all of which may help investigators understand the pathophysiology of TRD.
MRS may be useful for differentiating treatment responders from non-responders, for developing novel therapeutics that target the glutamatergic and GABA-ergic systems, and for understanding how depression treatments in general may affect brain metabolism. The sections below review TRD studies that examined neurotransmitter level differences between individuals with TRD and HVs as well as changes in neurotransmitter resulting from pharmacological and non-pharmacological interventions, with a focus on understanding which of these changes could be related to and predictive of successful treatment response. The reviewed non-pharmacological interventions include TMS and ECT, which is conducted by electrically stimulating the brain, and which can be performed unilaterally, bilaterally, or bifrontally (Kellner et al., 2012).
5.1. Neurotransmitter levels in TRD at baseline
Studies of GABA levels in TRD have mainly focused on cortical areas. Compared to HVs, individuals with TRD showed decreased GABA levels in the occipital cortex (Price et al., 2009; Sanacora et al., 2004), although no differences in GABA levels were observed in several other cortical areas, including the DLPFC (Baeken et al., 2017) and ACC (Persson et al., 2021; Price et al., 2009). Another study found that levels of Glx—a measure that combines glutamate and glutamine—were significantly lower in the left amygdala of treatment-resistant individuals with MDD, but not in HVs or in individuals with treatment-resistant bipolar disorder (Michael et al., 2003b). Another study found that Glx concentrations in the left DLPFC were significantly lower in individuals with TRD than in HVs (Baeken et al., 2017; Persson et al., 2021) but significantly higher in the left ACC (Pfleiderer et al., 2003). Other studies, however, reported no differences in the ACC between individuals with TRD and HVs with regard to Glx levels (Zheng et al., 2015) or glutamate levels (Evans et al., 2018a; Persson et al., 2021; Price et al., 2009). When GABA and Glx levels were compared between individuals with depression who responded to treatment and those with TRD, GABA levels were found to be decreased in the occipital cortex of those with TRD compared to treatment responders, but Glx levels did not differ between the two groups.
MRS studies have also focused on other neurotransmitters of interest. For instance, three studies examined levels of NAA—a brain metabolite linked to neuronal integrity and viability—in the amygdala, hippocampus, thalamus, and DLPFC. One study noted a significant decrease in NAA/Cr levels in the bilateral thalamus of individuals with TRD compared to HVs (Mu et al., 2007), and another study found lower NAA levels in the hippocampus of young individuals with TRD that inversely correlated with left hippocampal volume (Lefebvre et al., 2017). In contrast, other studies reported no differences in NAA levels measured in the amygdala, hippocampus (Michael et al., 2003b), and DLPFC (Zheng et al., 2015) of individuals with TRD compared to HVs.
Finally, two MRS studies examined m-Ino, Cho, and Cr levels. Zheng and colleagues found reduced m-Ino levels in the left lateral PFC of individuals with TRD compared to HVs, but no differences in the amygdala or hippocampus; in addition, no differences in Cho or Cr levels were observed in any of these brain regions (Zheng et al., 2015). Another study found that Cho levels were significantly increased in the DLPFC of adolescents with TRD compared to HVs (Zheng et al., 2010).
5.2. Non-pharmacological interventions
In MRS studies, increased Glx levels have been linked to successful response to ECT. Specifically, when the effects of ECT were examined in the amygdala and hippocampus of individuals with TRD, treatment response was associated with increased Glx in those brain areas (Michael et al., 2003a). The same study also identified increased Glx levels at the DLPFC following ECT treatment. Glx/Cr increases were also identified after 15 days of ECT and were positively correlated with increased brain volume in the medial temporal lobe and the right pregenual ACC.
With regard to TMS, one study found that Glx levels significantly increased in the DLPFC after 4 weeks of daily, repeated TMS treatments (Godfrey et al., 2021). Another study observed an increased glutamate/glutamine ratio in the DLPFC and ACC of individuals with TRD following TMS; interestingly, levels remained increased after a six-month follow-up period (Croarkin et al., 2016). The Glx/NAA ratio also increased in the left caudal middle frontal area following continuous but not intermittent TMS (Spurny-Dworak et al., 2022). However, another study found that Glx levels in the DLPFC remained unaltered after only 4 days of TMS (Baeken et al., 2017). GABA levels also appear to increase following successful TMS treatment (Baeken et al., 2017; Levitt et al., 2019), though one study reported no change in GABA levels in the DLPFC (Godfrey et al., 2021).
ECT and TMS studies have also examined NAA levels and how those levels change before and after treatment. Because NAA levels are considered a marker of neuronal health and integrity, changes in NAA concentrations after ECT and TMS could offer some insight into the mechanisms underlying successful response to these treatments. In individuals with TRD who responded positively to ECT, NAA levels in the amygdala and hippocampus increased compared to baseline (Michael et al., 2003b); furthermore, ECT response was associated with decreased NAA/Cr concentrations that positively correlated with increased medial temporal lobe volume (Cano et al., 2017). In another study of individuals with TRD who responded to TMS, higher levels of NAA were identified in the left ACC that were positively correlated with improvement in executive function; in contrast, no metabolite changes were observed in TRD participants who did not respond to TMS (Zheng et al., 2015). Fig. 3 provides a schematic representation of the structural MRS findings in individuals with TRD compared to HVs.
FIG. 3.
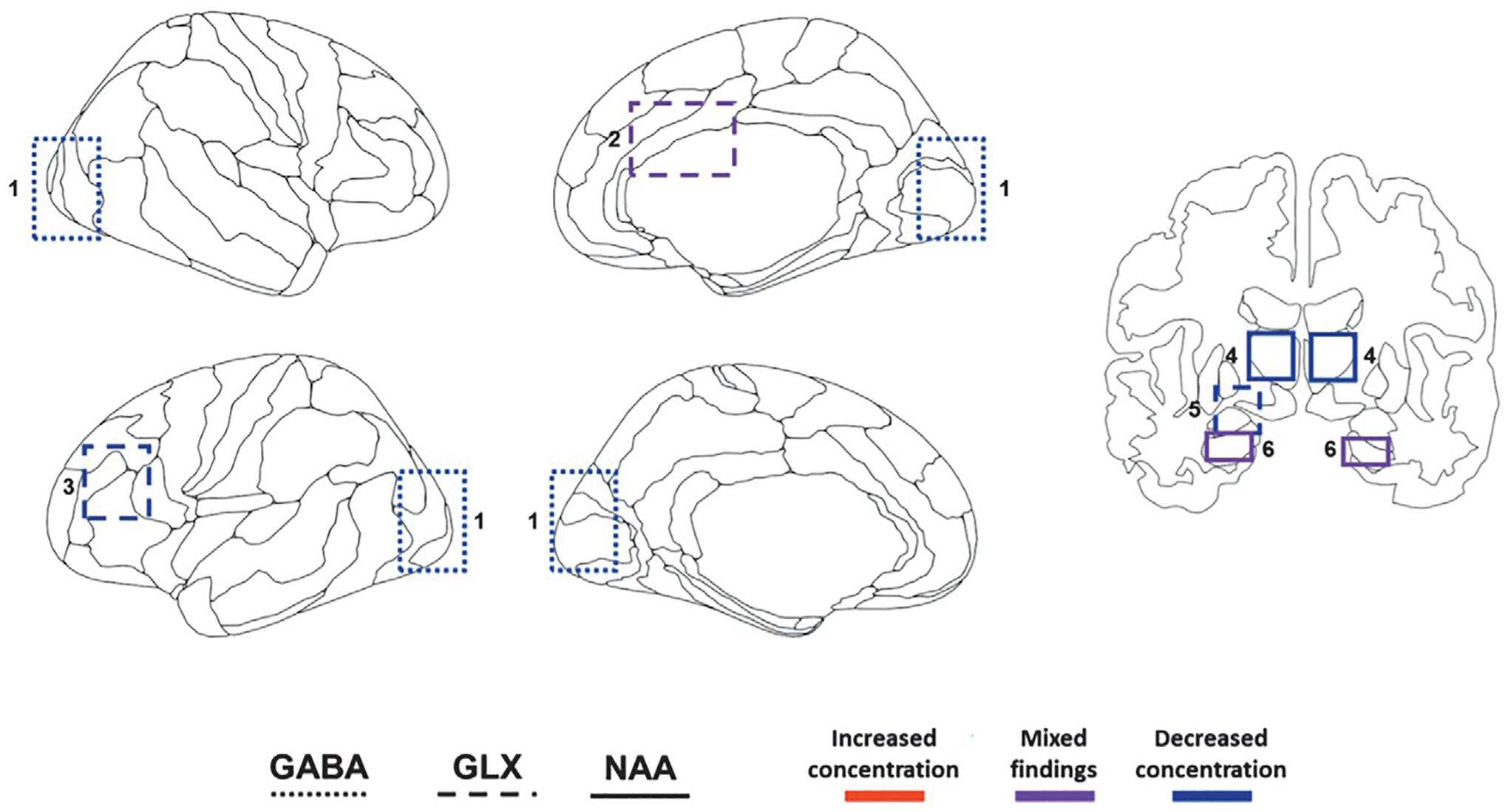
Schematic displaying general regions of interest in magnetic resonance spectroscopy (MRS) research in treatment-resistant depression (TRD) at baseline. 1: occipital cortex; 2: anterior cingulate cortex (ACC); 3: dorsolateral prefrontal cortex (DLPFC); 4: thalamus; 5: left amygdala, 6: hippocampus. Increases and decreases in metabolite concentration are with respect to healthy volunteers (HVs). GABA: Gamma-aminobutyric acid; Glx: Glutamine/glutamate; NAA: N-acetyl-aspartate.
5.3. Pharmacological interventions
Glx and GABA levels were assessed in the mPFC of individuals with TRD who received a single ketamine infusion (0.5mg/kg). In one study, increased Glx and GABA levels were observed during ketamine infusion compared to baseline (Milak et al., 2016). Another study of individuals with TRD found that peak GABA levels in the ACC correlated with reduced depressive symptoms 24 hours post-ketamine infusion (Singh et al., 2021). However, another double-blind, placebo-controlled study found no changes between the ketamine and placebo groups when neurotransmitter levels were assessed 24 hours post-ketamine infusion, suggesting that ketamine’s effects might be attenuated over time (Evans et al., 2018a).
Finally, another MRS study examined Cr levels before and after the administration of creatinine monohydrate (CM) as an augmentation therapy to selective serotonin reuptake inhibitors (SSRIs). Individuals with TRD who had not responded to the SSRI fluoxetine after 8 weeks of treatment received CM in addition to fluoxetine, which increased Cr levels in the frontal lobe (Kondo et al., 2011, 2016). MRS studies in TRD are summarized in Table 3.
Table 3.
Spectroscopy MRI studies in TRD.
| Article | Imaging modality | Aims | Experimental design | Summary of findings |
|---|---|---|---|---|
| Mervaala et al. (2000) | 1.5T 1H-MRS (PRESS) | To examine metabolite concentrations in mesial temporal lobe structures in patients with TRD and HVs | TRD group: N = 34 (18 female, mean age = 42.2 HV group (MRS): N = 12 (6 female, mean age = 33.8) |
An elevated choline/creatine intensity ratio was observed in the left mesial temporal lobe in patients with TRD compared to HVs. |
| Michael et al. (2003a) | 1.5T 1H-MRS (STEAM) | To investigate whether depression is accompanied by altered glutamate levels in the left DLPFC in a sample of patients with TRD with melancholic features before and after ECT | TRD group: N = 12 (8 female, mean age = 63.4) HV group: N = 12 (6 female, mean age = 62.0) |
Compared to HVs, patients with TRD had reduced Glx levels in the DLPFC. Longer depression history and more severe symptomatology correlated with lower NAA values. Cr levels also correlated with depression severity. Following ECT, responders (N = 8) demonstrated a marked increase in Glx such that they were no longer statistically distinct from HVs. Glx levels correlated with improved MADRS scores. |
| Pfleiderer et al. (2003) | 1.5T 1H-MRS (STEAM) | To assess the effect of successful ECT on Glx levels in the ACC in individuals with TRD | TRD group: N = 17 (12 female, mean age = 61.0) HV group: N = 17 (12 female, mean age = 60.1) |
Prior to ECT, Glx was significantly reduced in the left ACC in patients with TRD compared to HVs. Glx levels increased in responders (N = 12), such that they were no longer statistically distinct from HVs. |
| Sanacora et al. (2004) | 2.1T 1H-MRS | To replicate previous work demonstrating reduced GABA levels in the occipital cortex and extend to include other metabolites such as glutamate and potential clinical correlates in individuals with a wide range of depression severity, including TRD | MDDa group: N = 33 (11 female, mean age = 41.9) HV group: N = 38 (23 female, mean age = 35.7) |
Reduced GABA levels in the occipital cortex of individuals with MDD was replicated. In addition, concentrations of glutamate and GABA/glutamate were increased in the occipital cortex. Reduced GABA levels and increased glutamate levels were associated with melancholic and psychotic features. |
| Mu et al. (2007) | 1.5T 1H-MRS (PRESS) | To examine potentially altered metabolism in the thalamus of individuals with TRD | TRD group: N = 20 (10 female, mean age = 35.5) HV group: N = 20 (10 female, mean age = 36.8) |
NAA/Cr concentrations were decreased in both thalami of individuals with TRD compared to HVs, which may have been due to damage and loss of neurons. |
| Price et al. (2009) | 3T 1H-MRS (PRESS) | To compare GABA and Glx levels in the occipital cortex and ACC of individuals with TRD, non-treatment-resistant MDD (nTRD), and HVs | TRD group: N = 15 (7 female, mean age = 46.8) nTRD group: N = 18 (6 female, mean age = 38.3) HV group: N = 24 (13 female, mean age = 37.3) |
GABA levels in the occipital cortex were reduced in individuals with TRD compared to both nTRD and HV samples and could not be explained by any clinical or demographic factor. Reduced GABA levels in the ACC in individuals with TRD did not survive multiple comparison correction. |
| Zheng et al. (2015) | 3T 1H-MRS | To examine if altered neural metabolism in the ACC was altered by rTMS treatment and if these changes are associated with executive functioning in individuals with TRD | TRD group: N = 34 (11 female, mean age = 26.9) HV group: N = 28 (12 female, mean age = 27.6) |
NAA and Cho levels were reduced in the left ACC in individuals with TRD compared to HVs. Responders to active rTMS showed increased NAA levels in the left ACC. Higher NAA levels predicted greater executive functioning following active rTMS. |
| Croarkin et al. (2016) | 3T 1H-MRS (PRESS, 2DJ) | To examine changes in Gln/Glu concentrations in the ACC and left DLPFC in adolescents with TRD undergoing high frequency rTMS | TRD group: N = 10 (4 female, mean age = 15.4) | Increases in Gln/Glu concentrations in the ACC and left DLPFC occurred following up to 30 sessions of high frequency rTMS. Statistical significance was only reached with PRESS and not 2DJ. Depression symptom severity (CDRS-R) had an inverse relationship with Gln/Glu concentrations throughout the follow-up period. |
| Milak et al. (2016) | 3T 1H-MRS | To replicate, in humans with MDD, previous work demonstrating surges in glutamatergic compounds in the mPFC following ketamine administration observed in rodents | MDDb group: N = 11 (8 female, mean age = 38.8) | Increased Glx and GABA concentrations were observed in the mPFC following IV ketamine administration. |
| Baeken et al. (2017) | 3T 1H-MRS (PRESS) | To examine the effects of accelerated high frequency rTMS on GABAergic and glutamatergic metabolite concentrations and cellular integrity in the left DLPFC, right DLPFC, and rostral ACC in individuals with TRD | TRD group: N = 18 (12 female, mean age = 47.2) HV group: N = 18 (12 female, mean age = 45.8) |
At baseline, individuals with TRD exhibited decreased Glx concentrations in the left DLPFC compared to HVs. GABA concentration increases in the left DLPFC were associated with beneficial clinical outcome. Cellular integrity was not impacted by accelerated high frequency rTMS. |
| Lefebvre et al. (2017) | 3T 1H-MRS (PRESS) | To investigate the relationship between hippocampal NAA, glutamate concentrations and volume, and depressive symptoms in youth with TRD | TRD group: N = 18 (10 female, mean age = 17.9) HV group: N = 15 (8 female, mean age = 19.0) |
Youth with TRD exhibited lower NAA concentrations in the left hippocampus and greater Glx concentrations in the right hippocampus. Left NAA and left hippocampal volume were inversely correlated. In the right hippocampus only, NAA and Glx concentrations were tightly coupled. In the left hippocampus only, Glx concentrations were inversely correlated with age of onset. |
| Cano et al. (2017) | 3T 1H-MRS (PRESS) | To examine longitudinal hippocampal metabolite concentrations and the association between imaging changes and clinical improvement over the course of ECT treatment in individuals with TRD | TRD group: N = 12 (6 female, mean age = 59.2) HV group: N = 10 (5 female, mean age = 54.4) |
Left MTL volume increase was associated with hippocampal NAA/Cr concentration decreases and Glx/Cr increases following ECT in individuals with TRD. |
| Evans et al. (2018a) | 7T 1H-MRS (PRESS) | To examine glutamate levels in the pregenual ACC following ketamine administration and other metabolite changes, correlates, or predictors of antidepressant response | TRD group: N = 20 (12 female, mean age = 36.2) HV group: N = 17 (12 female, mean age = 34.7) |
No significant differences were observed between groups or between scanning sessions (baseline versus 24hour-post); however, there was a trend towards increased glutamate concentrations post-ketamine infusion in individuals with TRD. |
| Levitt et al. (2019) | 3T 1H-MRS (MEGA-PRESS) | To investigate whether rTMS administered to the left DLPFC resulted in increased GABA levels and whether these changes varied in responders compared to non-responders | TRD group: N = 26 (13 female, mean age = 38.4) | Increased GABA concentrations in the left DLPFC were statistically significant for the group who responded to rTMS treatment. |
| Godfrey et al. (2021) | 3T 1H-MRS (MEGA-PRESS) | To investigate the impact of rTMS stimulation in the left DLPFC on GABA and Glx levels at the stimulation site compared to M1 (control site) and the relationship of metabolite concentrations to depressive symptomatology at baseline and following treatment and their predictive value in individuals with TRD (bipolar and unipolar) | TRD groupc: N = 27 (11 female, mean age = 41.6) | No differences in GABA concentrations were observed following rTMS treatment. Although not correlated with antidepressant response, Glx levels increased significantly in the left DLPFC and right M1, suggesting that rTMS has potential non-specific treatment effects. |
| Singh et al. (2021) | 3T fMRS (MEGA-PRESS) | To explore the relationship between GABA and glutamate levels in the ACC and changes in depressive symptoms following a single IV ketamine infusion in individuals with TRD | TRD group: N = 7 (4 female, mean age = 45.0) | A significant peak in GABA levels in the ACC during the ketamine infusion was associated with next day remission and degree of clinical response. An increased NAA+NAAG peak in the ACC was also associated with remission. |
| Spurny-Dworak et al. (2022) | 3T 3D-multivoxel MRS (MEGALASER), surface-based MRS | To investigate the therapeutic effects of TBS on GABAergic and glutamatergic systems, and to examine changes in cortical neurotransmitter levels in individuals with TRD | TRD group: N = 12 (5 female, mean age = 35.0) | Glx/tNAA concentrations increased in the region of excitatory TBS treatment in individuals with TRD. |
ACC: anterior cingulate cortex; CDRS-R: Children’s Depression Rating Scale-Revised; Cho: choline; Cr: creatinine; DLPFC: dorsolateral prefrontal cortex; ECT: electroconvulsive therapy; GABA: gamma aminobutyric acid; Gln: glutamine; Glu: glutamate; Glx: glutamine/glutamate; HV: healthy volunteer; MADRS: Montgomery-Asberg Depression Rating Scale; mPFC: medial prefrontal cortex; MRS: magnetic resonance spectroscopy; MTL: medial temporal lobe; NAA: N-acetyl aspartate; NAAG: N-acetyl-aspartyl-glutamate; PRESS: point-resolved spectroscopy; rTMS: repetitive transcranial magnetic stimulation; STEAM: stimulated echo acquisition mode; TBS: theta-burst stimulation; tNAA: total N-acetyl-aspartate; TRD: treatment-resistant depression.
Mixed sample with some patients treated for TRD.
The group included TRD patients but TRD was not a requirement.
Both with bipolar and unipolar depression.
6. Insights, challenges, and future steps
The imaging studies reviewed above highlight the complexity of TRD. Brain areas that play a key role in emotional (amygdala) and reward (striatal areas) processing and that are part of brain networks that include the DMN and the SN appear to display altered activity, connectivity, metabolism, and structure in individuals with TRD compared to HVs and, often, to individuals with MDD who responded to treatment. Notably, when findings across different imaging modalities are integrated, a picture of deficits in TRD emerges that almost consistently reflects decreased connectivity and metabolite concentrations in frontal brain areas. However, firm conclusions regarding the direction of these changes and the reproducibility of these findings remain difficult to draw, largely because the number of studies that have focused on TRD is relatively small, and because several studies report contradictory findings. Another complicating factor is that existing hypotheses examined by imaging individuals with TRD derive from known deficits in brain function, metabolism, and structure observed in individuals with depression who responded to treatment. Such studies have thus not only influenced the focus of TRD research by guiding the selection of brain regions to examine but also by guiding the methodology that these studies employ, including the selection of fMRI tasks and the analytical approaches.
Non-pharmacological interventions such as ECT and TMS, as well as novel rapid-acting antidepressants such as ketamine and psilocybin effectively alleviate depressive symptoms in individuals with TRD. Imaging findings from studies that examined the efficacy of these interventions found that these agents can reverse some of the changes in brain activity, connectivity, metabolism, and structure identified in individuals with TRD at baseline versus post-treatment. However, to date, such research has offered very little insight into the neuronal and molecular mechanisms that underlie these interventions, leaving many questions about these treatments unanswered. Some of these questions concern the duration of the effect that these interventions exert and how the brain changes that they produce develop over time. It also remains unclear whether response to these treatments could be predicted based on specific patterns of brain function, metabolism, and structure in individuals with TRD.
At least some of the unanswered questions surrounding TRD and how pharmacological and non-pharmacological interventions improve symptoms in this type of depression are due to the particular challenges of TRD research. First, the specialized TRD diagnosis makes it difficult to enroll large patient populations. Second, the long-term treatment of TRD with multiple antidepressants and their poorly characterized effects on the brain introduce extra heterogeneity into this population over time. Specifically, because the long-term neural effects of antidepressants are not well characterized, these perturbatory effects increase the difficulty of determining the underlying features of TRD. Third, while the existing variability in the acquisition and tasks that imaging studies often employ reflects the innovative nature of these research studies, it also hinders our ability to directly compare results across these small studies. Finally, the relationship between clinical rating scales and imaging metrics has not firmly been established; as a result, it is difficult to map specific symptoms to patterns of brain activity and metabolism and to identify treatment targets that could help resolve specific TRD symptoms.
Several solutions could address at least some of these issues. For instance, data from smaller, currently available cohort studies could be combined in multi-variate models that may have important predictive value. Alternately, or in addition to this approach, large multi-site studies that could assemble large data sets (Spellman and Liston, 2020) could also help increase the power and reliability of imaging findings, although they face the challenge of coordinating data collection from multiple sites while ensuring data quality and consistency. In addition, developing standardized batteries and protocols could help address some of the issues of data sharing across different studies. These steps could help advance collaborative research and lead to more robust finding in imaging TRD research that could improve our understanding of TRD and spur development of novel interventions and treatment targets.
Acknowledgments
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Funding
Funding for this work was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857). The work was completed as part of the authors’ official duties as Government employees. The views expressed do not necessarily reflect the views of the NIH, the Department of Health and Human Services, or the United States Government.
Footnotes
Conflict of interest
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- Baeken C, Lefaucheur J-P, Van Schuerbeek P, 2017. The impact of accelerated high frequency rTMS on brain neurochemicals in treatment-resistant depression: insights from 1H MR spectroscopy. Clin. Neurophysiol 128, 1664–1672. [DOI] [PubMed] [Google Scholar]
- Bratsos S, Saleh SN, 2019. Clinical efficacy of ketamine for treatment-resistant depression. Cureus 11, e5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M, Martínez-Zalacaín I, Bernabéu-Sanz Á, Contreras-Rodríguez O, Hernández-Ribas R, Via E, De Arriba-Arnau A, Gálvez V, Urretavizcaya M, Pujol J, Menchón JM, Cardoner N, Soriano-Mas C, 2017. Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment-resistant depression: a longitudinal neuroimaging study. Transl. Psychiatry 7, e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, Tanner M, Kaelen M, Mcgonigle J, Murphy K, Leech R, Curran HV, Nutt DJ, 2017. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep 7, 13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-H, Lin W-C, Tu P-C, Li C-T, Bai Y-M, Tsai S-J, Su T-P, 2019. Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: A double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J. Affect. Disord 259, 15–20. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Wall CA, Murphy LL, Sampson SM, Frye MA, Port JD, 2016. Transcranial magnetic stimulation potentiates glutamatergic neurotrans-mission in depressed adolescents. Psychiatry Res. Neuroimaging 247, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Diego-Adeliño J, Pires P, Gómez-Ansón B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, Martín-Blanco A,Álvarez E, Pérez V, Portella MJ, 2014. Micro-structural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol. Med 44, 1171–1182. [DOI] [PubMed] [Google Scholar]
- De Kwaasteniet BP, Rive MM, Ruhé HG, Schene AH, Veltman DJ, Fellinger L, Van Wingen GA, Denys D, 2015. Decreased resting-state connectivity between neurocognitive networks in treatment resistant depression. Front. Psychol 6, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JW, Lally N, An L, Li N, Nugent AC, Banerjee D, Snider SL, Shen J, Roiser JP, Zarate CA, 2018a. 7T 1H-MRS in major depressive disorder: a ketamine treatment study. Neuropsychopharmacology 43, 1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA, 2018b. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol. Psychiatry 84, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J, Eisendrath SJ, Fryer SL, Gillung E, Roach BJ, Mathalon DH, 2017. Blunted amygdala activity is associated with depression severity in treatment-resistant depression. Cogn. Affect. Behav. Neurosci 17, 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado CP, Maller JJ, Fitzgerald PB, 2008. A magnetic resonance imaging study of the entorhinal cortex in treatment-resistant depression. Psychiatry Res. Neuroimaging 163, 133–142. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Lloyd SW, Lux L, Gartlehner G, Hansen RA, Brode S, Jonas DE, Evans TS, Viswanathan M, Lohr KN, 2014. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J. Clin. Psychiatry 75, 477–489. [DOI] [PubMed] [Google Scholar]
- Ge R, Blumberger DM, Downar J, Daskalakis ZJ, Dipinto AA, Tham JCW, Lam R, Vila-Rodriguez F, 2017. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment resistant depression: a pilot study. J. Affect. Disord 218, 75–81. [DOI] [PubMed] [Google Scholar]
- Ge R, Downar J, Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F, 2020. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimul 13, 206–214. [DOI] [PubMed] [Google Scholar]
- Godfrey KEM, Muthukumaraswamy SD, Stinear CM, Hoeh N, 2021. Effect of rTMS on GABA and glutamate levels in treatment-resistant depression: an MR spectroscopy study. Psychiatry Res. Neuroimaging 317, 111377. [DOI] [PubMed] [Google Scholar]
- Godfrey KEM, Muthukumaraswamy SD, Stinear CM, Hoeh N, 2022. Decreased salience network fMRI functional connectivity following a course of rTMS for treatment-resistant depression. J. Affect. Disord 300, 235–242. [DOI] [PubMed] [Google Scholar]
- Guo W-B, Liu F, Chen J-D, Xu X-J, Wu R-R, Ma C-Q, Gao K, Tan C-L, Sun X-L, Xiao C-Q, Chen H-F, Zhao J-P, 2012. Altered white matter integrity of forebrain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 38, 201–206. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Rosenbaum JF, Alpert JE, 2015. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin. Neurosci 17, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM, 2012. ECT in treatment-resistant depression. Am. J. Psychiatry 169, 1238–1244. [DOI] [PubMed] [Google Scholar]
- Kelly CA, Freeman KB, Schumacher JA, 2022. Treatment-resistant depression with anhedonia: integrating clinical and preclinical approaches to investigate distinct pheno-types. Neurosci. Biobehav. Rev 136, 104578. [DOI] [PubMed] [Google Scholar]
- Klok MPC, Van Eijndhoven PF, Argyelan M, Schene AH, Tendolkar I, 2019. Structural brain characteristics in treatment-resistant depression: review of magnetic resonance imaging studies. BJPsych. Open 5, e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo DG, Sung Y-H, Hellem TL, Fiedler KK, Shi X, Jeong E-K, Renshaw PF, 2011. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord 135, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo DG, Forrest LN, Shi X, Sung Y-H, Hellem TL, Huber RS, Renshaw PF, 2016. Creatine target engagement with brain bioenergetics: a dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids 48, 1941–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V, Brammer MJ, Poon L, Simmons A, Williams SCR, Checkley SA, Sharma T, 2003. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biol. Psychiatry 54, 777–791. [DOI] [PubMed] [Google Scholar]
- Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M, 2016. Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimul 9, 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre D, Langevin LM, Jaworska N, Harris AD, Lebel RM, Jasaui Y, Kirton A, Wilkes TC, Sembo M, Swansburg R, Macmaster FP, 2017. A pilot study of hippocampal N-acetyl-aspartate in youth with treatment resistant major depression. J. Affect. Disord 207, 110–113. [DOI] [PubMed] [Google Scholar]
- Lener MS, Iosifescu DV, 2015. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann. N. Y. Acad. Sci 1344, 50–65. [DOI] [PubMed] [Google Scholar]
- Levitt JG, Kalender G, O’neill J, Diaz JP, Cook IA, Ginder N, Krantz D, Minzenberg MJ, Vince-Cruz N, Nguyen LD, Alger JR, Leuchter AF, 2019. Dorsolateral prefrontal γ-aminobutyric acid in patients with treatment-resistant depression after transcranial magnetic stimulation measured with magnetic resonance spectroscopy. J. Psychiatry Neurosci 44, 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yücel M, 2009. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J. Affect. Disord 117, 1–17. [DOI] [PubMed] [Google Scholar]
- Loureiro JRA, Leaver A, Vasavada M, Sahib AK, Kubicki A, Joshi S, Woods RP, Wade B, Congdon E, Espinoza R, Narr KL, 2020. Modulation of amygdala reactivity following rapidly acting interventions for major depression. Hum. Brain Mapp 41, 1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro JRA, Sahib AK, Vasavada M, Leaver A, Kubicki A, Wade B, Joshi S, Hellemann G, Congdon E, Woods RP, Espinoza R, Narr KL, 2021. Ketamine’s modulation of cerebro-cerebellar circuitry during response inhibition in major depression. Neuroimage Clin 32, 102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S-X, Zhang L, Wang R, Zhao H, Liu C, 2019. A resting-state study of volumetric and functional connectivity of the habenular nucleus in treatment-resistant depression patients. Brain Behav 9, e01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RCK, Huang X, Kemp GJ, Mechelli A, Gong Q, 2011. Resting-state functional connectivity in treatment-resistant depression. Am. J. Psychiatry 168, 642–648. [DOI] [PubMed] [Google Scholar]
- Ma C, Ding J, Li J, Guo W, Long Z, Liu F, Gao Q, Zeng L, Zhao J, Chen H, 2012. Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS One 7, e45263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machino A, Kunisato Y, Matsumoto T, Yoshimura S, Ueda K, Yamawaki Y, Okada G, Okamoto Y, Yamawaki S, 2014. Possible involvement of rumination in gray matter abnormalities in persistent symptoms of major depression: an exploratory magnetic resonance imaging voxel-based morphometry study. J. Affect. Disord 168, 229–235. [DOI] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Thomson RHS, Daigle M, Barr MS, Fitzgerald PB, 2012. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil’s in de-tail. Hippocampus 22, 9–16. [DOI] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Mertens LJ, Wall MB, Roseman L, Demetriou L, Nutt DJ, Carhart-Harris RL, 2020. Therapeutic mechanisms of psilocybin: changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J. Psychopharmacol 34, 167–180. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Föhr J, Könönen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamäki H, Karjalainen AK, Lehtonen J, 2000. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol. Med 30, 117–125. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B, 2003a. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol. Med 33, 1277–1284. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B, 2003b. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology 28, 720–725. [DOI] [PubMed] [Google Scholar]
- Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, Mao X, Rodriguez CI, Oquendo MA, Suckow RF, Cooper TB, Keilp JG, Shungu DC, Mann JJ, 2016. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol. Psychiatry 21, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtchian A, Evans JW, Kraus C, Yuan P, Kadriu B, Nugent AC, Roiser JP, Zarate C., a. J., 2021. Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol. Psychiatry 26, 3292–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Xie P, Yang Z-S, Yang D-L, Lv F-J, Luo T-Y, Li Y, 2007. 1H magnetic resonance spectroscopy study of thalamus in treatment resistant depressive patients. Neurosci. Lett 425, 49–52. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Collins KA, Fields J, Dewilde KE, Phillips ML, Mathew SJ, Wong E, Tang CY, Charney DS, Iosifescu DV, 2015. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl. Psychiatry 5, e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tomita M, Horikawa N, Ishibashi M, Uematsu K, Hiraki T, Abe T, Uchimura N, 2021. Functional connectivity between the amygdala and subgenual cingulate gyrus predicts the antidepressant effects of ketamine in patients with treatment-resistant depression. Neuropsychopharmacol. Rep 41, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H-J, Zheng H-R, Ning Y-P, Zhang Y, Shan B-C, Zhang L, Yang H-C, Liu J, Li Z-X, Zhou J-S, Zhang Z-J, Li L-J, 2013. Abnormalities of cortical-limbiccerebellar white matter networks may contribute to treatment-resistant depression: a diffusion tensor imaging study. BMC Psychiatry 13, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Wall A, Weis J, Gingnell M, Antoni G, Lubberink M, Bodén R, 2021. Inhibitory and excitatory neurotransmitter systems in depressed and healthy: a positron emission tomography and magnetic resonance spectroscopy study. Psychiatry Res. Neuroimaging 315, 111327. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, Fiebich M, Arolt V, Heindel W, 2003. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res 122, 185–192. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ, 2009. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol. Psychiatry 65, 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D, Xu G, Lu Z, Liang H, Lin K, Tang M, 2019. Comparative study of cognitive function between treatment-resistant depressive patients and first-episode depressive patients. Neuropsychiatr. Dis. Treat 15, 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Grajales AM, Salas R, Robinson ME, Qi K, Murrough JW, Mathew SJ, 2021. Habenula connectivity and intravenous ketamine in treatment-resistant depression. Int. J. Neuropsychopharmacol 24, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, Demetriou L, Wall MB, Nutt DJ, Carhart-Harris RL, 2018. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology 142, 263–269. [DOI] [PubMed] [Google Scholar]
- Runia N, Yücel DE, Lok A, De Jong K, Denys DAJP, Van Wingen GA, Bergfeld IO, 2022. The neurobiology of treatment-resistant depression: a systematic review of neuroimaging studies. Neurosci. Biobehav. Rev 132, 433–448. [DOI] [PubMed] [Google Scholar]
- Sahib AK, Loureiro JRA, Vasavada MM, Kubicki A, Wade B, Joshi SH, Woods RP, Congdon E, Espinoza R, Narr KL, 2020. Modulation of inhibitory control networks relate to clinical response following ketamine therapy in major depression. Transl. Psychiatry 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahib AK, Loureiro JR, Vasavada M, Anderson C, Kubicki A, Wade B, Joshi SH, Woods RP, Congdon E, Espinoza R, Narr KL, 2022. Modulation of the functional connectome in major depressive disorder by ketamine therapy. Psychol. Med 52, 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, Krystal JH, Mason GF, 2004. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry 61, 705–713. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M, 2012. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepens DS, Van Waarde JA, Lok A, De Vries G, Denys DAJP, Van Wingen GA, 2020. The link between structural and functional brain abnormalities in depression: a systematic review of multimodal neuroimaging studies. Front. Psychol 11, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Blasco M, Portella MJ, Gómez-Ansón B, De Diego-Adeliño J, Vives-Gilabert Y, Puigdemont D, Granell E, Santos A,Álvarez E, Pérez V, 2013. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br. J. Psychiatry 202, 434–440. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM, 1998. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression: controlled magnetic resonance imaging study. Br. J. Psychiatry 172, 527–532. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Glabus MF, Goodwin GM, Ebmeier KP, 2002. Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br. J. Psychiatry 180, 434–440. [DOI] [PubMed] [Google Scholar]
- Siegel JS, Palanca BJA, Ances BM, Kharasch ED, Schweiger JA, Yingling MD, Snyder AZ, Nicol GE, Lenze EJ, Farber NB, 2021. Prolonged ketamine infusion modulates limbic connectivity and induces sustained remission of treatment-resistant depression. Psychopharmacology 238, 1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH, 2008. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Port JD, Voort JLV, Coombes BJ, Geske JR, Lanza IR, Morgan RJ, Frye MA, 2021. A preliminary study of the association of increased anterior cingulate gamma-aminobutyric acid with remission of depression after ketamine administration. Psychiatry Res 301, 113953. [DOI] [PubMed] [Google Scholar]
- Spellman T, Liston C, 2020. Toward circuit mechanisms of pathophysiology in depression. Am. J. Psychiatry 177, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurny-Dworak B, Godbersen GM, Reed MB, Unterholzner J, Vanicek T, Baldinger-Melich P, Hahn A, Kranz GS, Bogner W, Lanzenberger R, Kasper S, 2022. The Impact of theta-burst stimulation on cortical GABA and glutamate in treatment-resistant depression: a surface-based MRSI analysis approach. Front. Mol. Neurosci 15, 913274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Maller JJ, Daskalakis ZJ, Furtado CC, Fitzgerald PB, 2009. Morphology of the corpus callosum in treatment-resistant schizophrenia and major depression: corpus callosum in schizophrenia and depression. Acta Psychiatr. Scand 120, 265–273. [DOI] [PubMed] [Google Scholar]
- Sun J, Ma Y, Guo C, Du Z, Chen L, Wang Z, Li X, Xu K, Luo Y, Hong Y, Yu X, Xiao X, Fang J, Lu J, 2023. Distinct patterns of functional brain network integration between treatment-resistant depression and non treatment-resistant depression: a resting-state functional magnetic resonance imaging study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 120, 110621. [DOI] [PubMed] [Google Scholar]
- Vasavada MM, Loureiro J, Kubicki A, Sahib A, Wade B, Hellemann G, Espinoza RT, Congdon E, Narr KL, Leaver AM, 2021. Effects of serial ketamine infusions on corticolimbic functional connectivity in major depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q-Z, Li D-M, Kuang W-H, Zhang T-J, Lui S, Huang X-Q, Chan RCK, Kemp GJ, Gong Q-Y, 2011. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum. Brain Mapp 32, 1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, 2013. Decreased hippocampal volume is related to white matter abnormalities in treatment-resistant depression. Int. J. Brain Disord. Treat 2, 12. [Google Scholar]
- Yüksel C, Öngür D, 2010. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol. Psychiatry 68, 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD, 2018. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 23, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T-J, Wu Q-Z, Huang X-Q, Sun X-L, Zou K, Lui S, Liu F, Hu J-M, Kuang W-H, Li D-M, Li F, Chen H-F, Chan RCK, Mechelli A, Gong Q-Y, 2009. Magnetization transfer imaging reveals the brain deficit in patients with treatment-refractory depression. J. Affect. Disord 117, 157–161. [DOI] [PubMed] [Google Scholar]
- Zheng H, Zhang L, Li L, Liu P, Gao J, Liu X, Zou J, Zhang Y, Liu J, Zhang Z, Li Z, Men W, 2010. High-frequency rTMS treatment increases left prefrontal myo-inositol in young patients with treatment-resistant depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 34, 1189–1195. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jia F, Guo G, Quan D, Li G, Wu H, Zhang B, Fan C, He X, Huang H, 2015. Abnormal anterior cingulate N-acetylaspartate and executive functioning in treatment-resistant depression after rTMS therapy. Int. J. Neuropsychopharmacol 18, pyv059. [DOI] [PMC free article] [PubMed] [Google Scholar]


