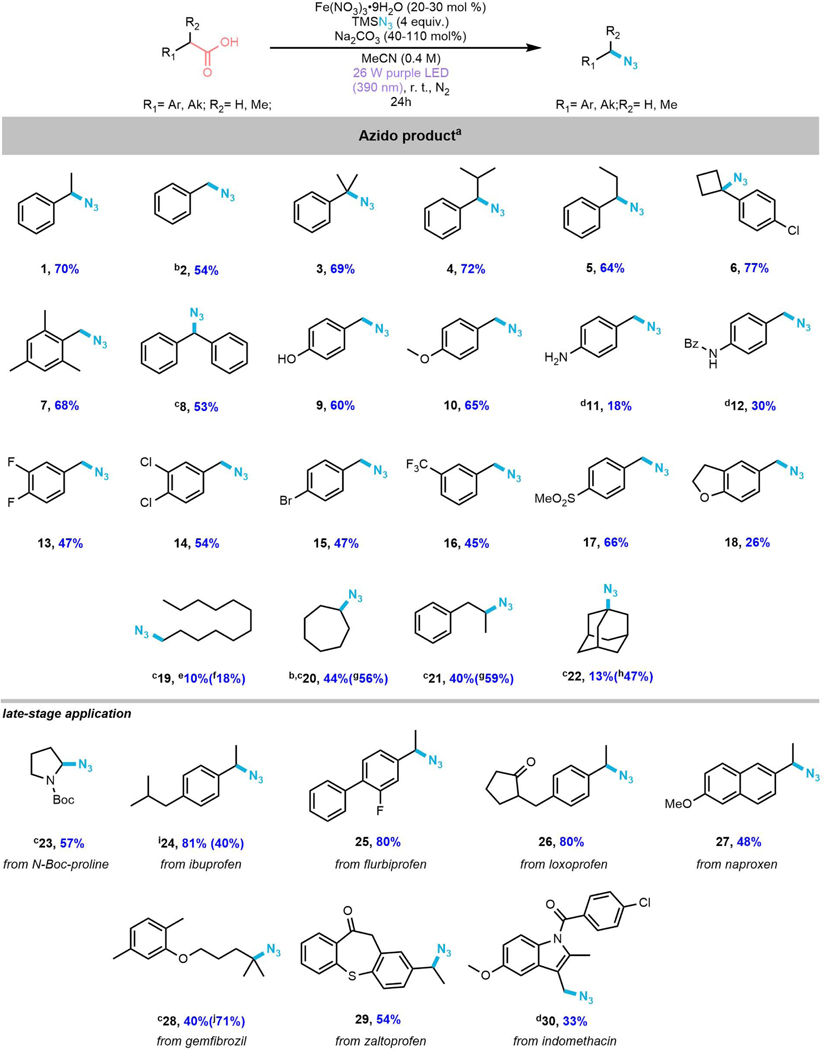
Figure 3. Scope of photocatalytic decarboxylative azidation of carboxylic acidsa.
aStandard conditions: Substrate (0.1 mmol), Fe(NO3)3·9H2O (0.02 mmol), TMSN3 (0.4 mmol), Na2CO3 (1.1 mmol), MeCN (0.25 mL), 390 nm LED light, rt, 24 h. bCH2Br2 was added as an internal standard (NMR yield).
cFe(NO3)3·9H2O (0.03 mmol) was used.
dNa2CO3 (0.04 mmol) was used.
eReaction performed on 0.3 mmol scale with (2 X 52W) purple LEDs (390 nm).
fReaction was performed under the following conditions: Substrate (0.1 mmol), Fe(NO3)3·9H2O (0.03 mmol), TMSN3 (0.4 mmol), Na2CO3 (0.02 mmol), MeCN/DCM=1/1 (0.25 mL), Mg(NO3)2·6H2O (0.1 mmol), 390 nm LED light, rt, 24 h. NMR yield is in the parentheses (CH2Br2 as an internal standard).
gsame conditions as f but MeCN (0.25 mL) was used. NMR yield is in the parentheses (CH2Br2 as an internal standard).
hsame conditions as f but Fe(NO3)3·9H2O (0.02 mmol) was used. NMR yield is in the parentheses (CH2Br2 as an internal standard).
iReaction performed on 500 mg scale and yield is in the parentheses.
j Same conditions as f but Fe(NO3)3·9H2O (0.02 mmol) and MeCN (0.25 mL) were used. NMR yield is in the parentheses (CH2Br2 as an internal standard).