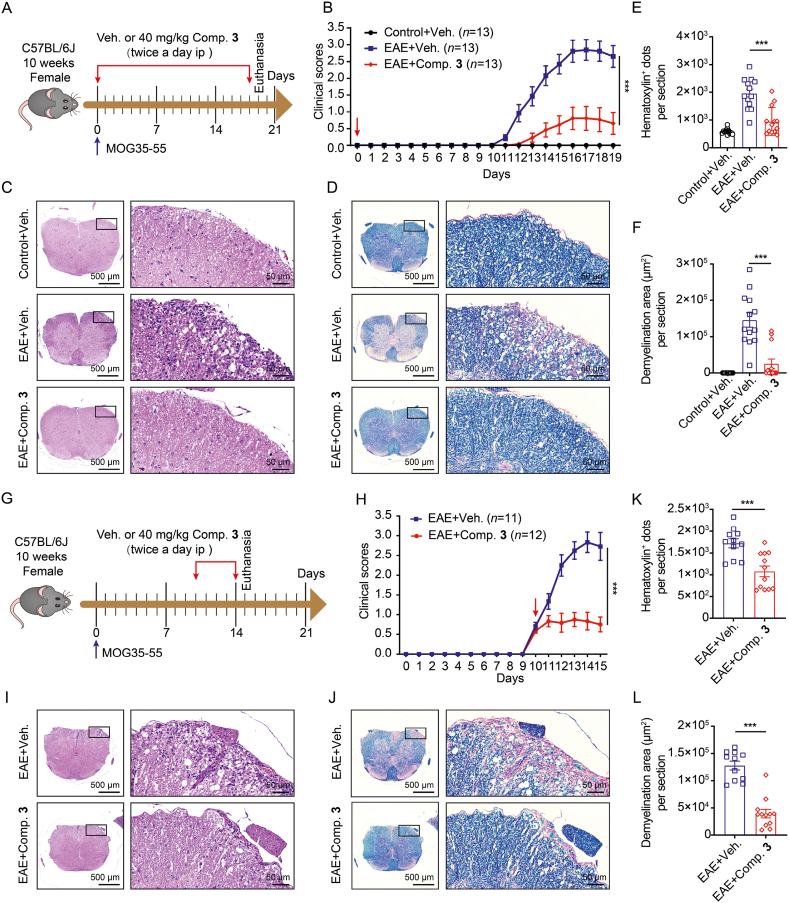
Figure 6.
Compound 3 alleviates the immunopathological damages of MOG35-55-elicited EAE. (A–F) Suppression of EAE disease on treatment with compound 3 from beginning Day 0 to Day 18 post-MOG35-55 vaccination. (A) Experimental schematic of MOG35-55-elicited EAE model. (B) Clinical scores. (C and E) Illustrative hematoxylin and eosin (H&E) images of the spinal cord and statistical histograms for hematoxylin positive dots. (D and F) Illustrative luxol fast blue (LFB) staining images of the spinal cord and statistical histograms of the demyelination area. (G–L) EAE disease amelioration by compound 3 treatment from Day 10 to Day 14 post MOG35-55 immunization. (G) Experimental schematic of EAE model. (H) Clinical scores. (I–L) Representative H&E and LFB staining images of the spinal cord and statistical histograms. Veh. represents vehicle; Comp. 3 represents compound 3. Data were represented as mean ± SEM. ∗∗∗P < 0.001 vs. EAE + veh. group.