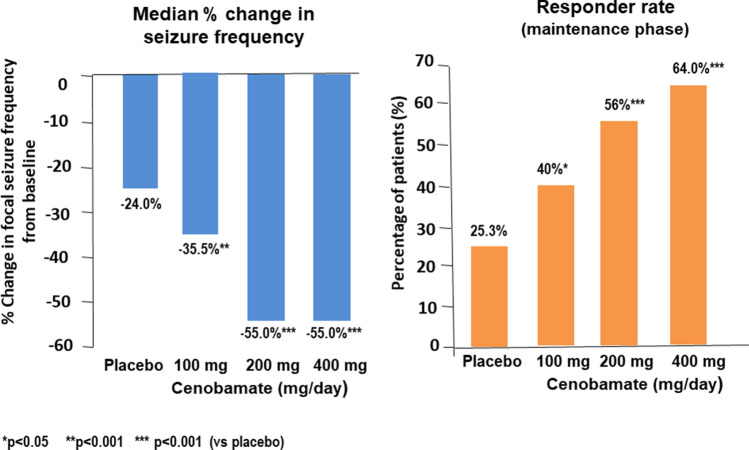
Fig. 3.
Efficacy outcomes for the two co-primary efficacy endpoints of the pivotal, placebo-controlled, adjunctive-therapy trial of cenobamate in adults with uncontrolled focal seizures. The median percent change in seizure frequency from the baseline endpoint was calculated in the modified intent-to-treat population (randomised patients who took at least one dose of the study medication and had any post-baseline seizure data), which included 106 patients for the placebo group and 108, 109 and 111 patients for the cenobamate 100-mg, 200-mg, and 400-mg dose groups, respectively. The responder rate endpoint (proportion of patients with at least a 50% reduction in seizure frequency from baseline) was calculated in the modified intent-to-treat maintenance phase population (randomised patients who took at least one dose of study medication in the maintenance phase and had any maintenance phase seizure data), which included 102 patients for the placebo group and 102, 98 and 95 patients for the cenobamate 100-mg, 200-mg, and 400-mg dose groups, respectively. Redrawn based on data from Krauss et al. [102]