Figure 1.
Interactors of unassembled chaperonin subunits destined for degradation
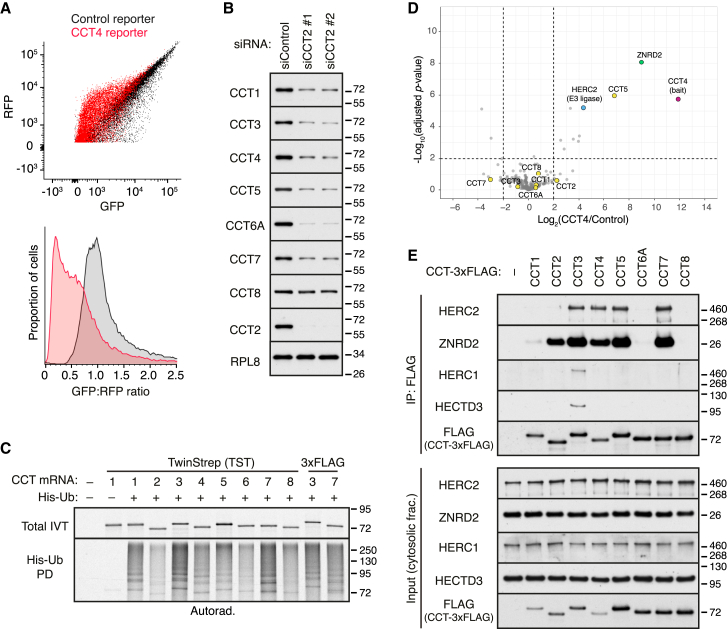
(A) HEK293T cells were transfected with a GFP-RFP dual-color reporter of orphan CCT4 degradation (red; see Figure S1A) or a control GFP-RFP reporter lacking the CCT4 insert (black), then analyzed by flow cytometry. CCT4 is fused to GFP, with RFP serving as a translation control. Shown are overlaid scatter plots of individual transfected cells (top) and the corresponding histograms of the GFP:RFP ratio (bottom).
(B) Cells were treated with control or CCT2-targeting small interfering RNAs (siRNAs) for 72 h, and total cell lysates were analyzed by immunoblotting for the proteins indicated on the left.
(C) CCT subunits containing a C-terminal TwinStrep tag (TST) or 3xFLAG tag were translated in rabbit reticulocyte lysate (RRL) containing 35S-methionine and His-tagged ubiquitin (His-Ub). Samples were analyzed directly (total IVT, in vitro translation) or after ubiquitin pull-down under denaturing conditions via the His-tag (His-Ub PD).
(D) CCT4-TST was translated in RRL, affinity-purified under native conditions, and analyzed by label-free quantitative mass spectrometry. Proteins in the upper right quadrant are significantly enriched with CCT4.
(E) Cells transiently expressing 3xFLAG-tagged CCT subunits were subjected to anti-FLAG immunoprecipitation (IP) under non-denaturing conditions. Input and IP samples were analyzed by immunoblotting for the indicated proteins.
See also Figures S1 and S2.