Figure 5.
Structural basis of orphan recognition by the ZNRD2-HERC2 complex
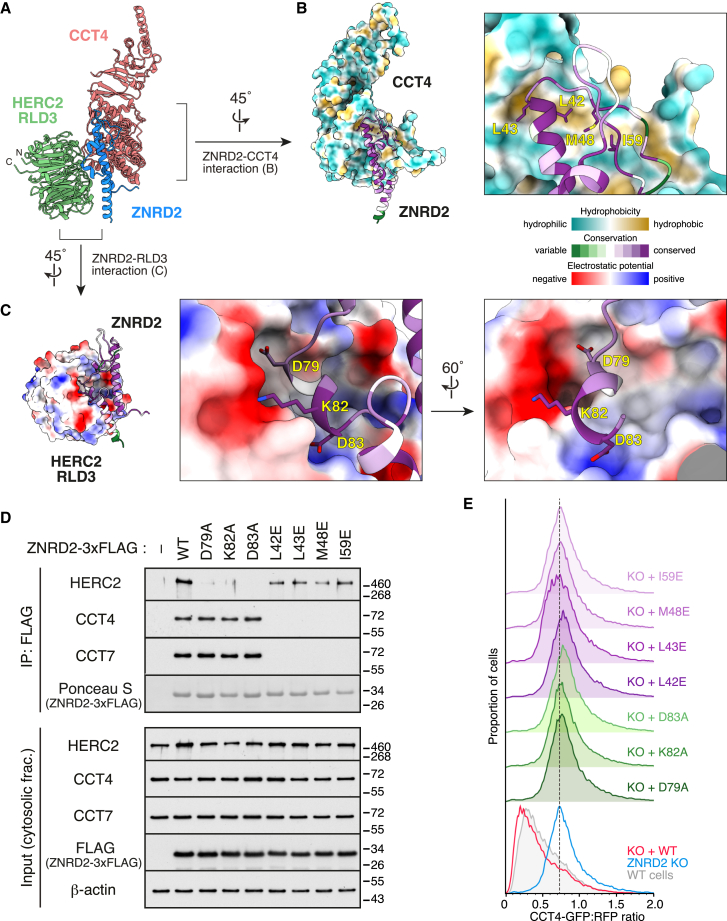
(A) AlphaFold-predicted model of a putative ternary complex consisting of CCT4 (pink), ZNRD2 (blue), and the RLD3 domain of HERC2 (green). The position of a C-terminal two-helix domain of ZNRD2 was not predicted with high confidence, so this element, the flexible linker attaching it to the N-terminal domain, and the unstructured N-terminal tail, are not shown.
(B) Interface between CCT4 and ZNRD2 in the predicted model. CCT4 is shown in surface representation and colored by hydrophobicity. ZNRD2 is in cartoon representation and colored by conservation. The close-up view shows four highly conserved residues of ZNRD2 that occupy a hydrophobic patch of CCT4 also found in other CCT subunits.
(C) Interface between ZNRD2 and RLD3 of HERC2 in the predicted model. RLD3 is shown in surface representation and colored by electrostatic potential, while ZNRD2 is shown as in (B). The close-up views show three highly conserved residues of ZNRD2 that mediate this predicted interaction.
(D) ZNRD2-3xFLAG constructs containing point mutations in the residues, highlighted in (B) and (C), were transiently expressed in ZNRD2-KO cells and analyzed for interactions with endogenous HERC2, CCT4, and CCT7 by non-denaturing IP with anti-FLAG affinity resin. Input and IP samples were subjected to immunoblotting as indicated.
(E) CCT4 reporter degradation was analyzed by flow cytometry in WT cells (filled gray), ZNRD2-KO cells (blue), and ZNRD2-KO cells transiently re-expressing WT (red) or mutant ZNRD2-3xFLAG constructs. The mutants are the same as those analyzed in (D).
See also Figures S6 and S7.